Structural Biochemistry/Inorganic Chemistry
Importance of Inorganic Chemistry in Biochemistry: Bioinorganic Chemistry
[edit | edit source]Although biochemistry generally focuses on the reactions and interactions of biological and organic molecules within the body, the roles and interactions of various inorganic molecules with the macromolecules in the body are just as important. Of all the elements known, only a few are essential for living organisms: Hydrogen (H), Carbon (C), Nitrogen (N), Oxygen (O), Sodium (Na), Phosphorus (P), Sulfur (S), Chlorine (Cl), Potassium (K), and Calcium (Ca). Additionally, a small amount of trace elements are essential for certain subsets of organisms. These elements include Lithium (Li), Beryllium (Be), the transition metals Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), Zinc (Zn), and Molybdenum (Mo), and the nonmetals Selenium (Se) and Iodine (I).
As a general overview, metal ions have varying roles in the body. Some examples of the metal ions’ roles are:
1)Linking distant residues or parts of the amino acids in the proteins together. 2)Mediating interactions between the protein ligand. 3)Positioning themselves in the active sites of the macromolecules as nucleophilic catalysts or as a key component in the electron transfer chain.
There are six major elements that are important. They provide the building blocks for nucleic acids, proteins, lipids, etc. They are:
- Sulfur:is a chemical element with a symbol S and its atomic number is 16. It is an abundant and multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8.
- Phosphorus:is a chemical element with a symbol P and its atomic number is 15. Also, it is a multivalent nonmetal of the nitrogen group. Phosphorus as a mineral is almost always present in its maximally oxidised state, as inorganic phosphate rocks.
- Nitrogen:is a chemical element with a symbol N and its atomic number is 7. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.09% by volume of Earth's atmosphere.
- Oxygen: is a chemical element with symbol O and its atomic number is 8.
- Carbon:is the chemical element with symbol C and its atomic number is 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons that is available to form covalent chemical bonds.
- Hydrogen:is a chemical element with a symbol H and its atomic number is 1. With an average atomic weight of 1.00794 u, hydrogen is the lightest element and its monatomic form is the most abundant chemical.
It would appear logical that numerous metal ions would be able to fulfill the biological processes. However, biological processes require a specific metal in order to proceed because of their unique combination of properties such as specific crystal field stabilization energy, complex conformation, and electron transition states. For example:
- coagulation cascade: Ca2+ ions
- protein biosynthesis: Mg2+ ions
- biomineralization (producing minerals to harden the existing tissues): Ca2+, magnesium, iron, etc.
- energy storage: phosphorous as inorganic phosphate Pi, Na+, K+, iron, etc.
- signaling: Ca2+, boron, nitrogen, oxygen
- Lewis acid-base catalysis: zinc, iron, manganese
- numerous proteins, oxidative processes, and enzymes: Zn2+ ion
A macromolecule is a very large molecule that has a polypeptide chain structure. Proteins, rubber, genes, polysaccarides, and synthetic polymers all consist of macromolecules. Macromolecules interact with each other and with small molecules. All of the interactions reflect the complementary between the interacting species. Sometimes, the complementary is general, as in the association of hydrophobic groups, but more often an exact fit of size, shape and chemical affinity is involved.
Inorganic macromolecules can be divided into several categories such as solids formed due to covalent bonds, organosilanes, siloxanes and organosiloxanes. Inorganic molecules are generally simple and are not normally found in living things. Although all organic substances contain carbon, some substances containing carbon, such as diamonds, are considered inorganic.
Read more: http://www.answers.com/topic/inorganic-molecules#ixzz2BnXuBZvS
Examples
[edit | edit source]Solids Formed by Covalent bonds
[edit | edit source]Diamond, graphite, silicon, and germanium are some examples of large inorganic macromolecules. However, Zinc sulfide has two forms/phases: the wurtzite and the zinc blende.
<br?>
Wurtzite is a mineral that includes zinc sulfide (Fe,Zn)S.
ZnO, SiC, AlN, CaSe, BN, C(Hexagonal Diamond) all have the same crystal structure in terms of bonding, symmetry, and packing sequence.
Zinc blende is cubic. Its' structure has the same bonding skeleton as the diamond structure.
Wurtzite and zinc blende structures are the two common structural types of inorganic macromolecules.
Elastic Sulfur
[edit | edit source]When liquid sulfur is poured into cold water, a long chain of -S-S-S-S-S- is formed. This phase is known as the elastic sulfur phase.
Nucleic Acids
[edit | edit source]Nucleic acids are polymers made of nucleotides connected via phosphate-sugar backbones. There are two kinds of nucleic acids ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). A nucleotide is made up of a 5-membered,sugar (ribose or deoxyribose) which is connected to a nitrogenous base (guanine, adenine, thymine, cytosine, uracil) and phosphorous group(s). DNA is made up of nucleotides containing guanine, adenine, thymine, and cytosine bases. RNA is made up of nucleotides containing guanine, adenine, uracil, and cytosine bases. The nucleotides are attached to each other via a phosphate group along the 5'-3' carbon side of the sugar. In addition, DNA is usually a double-stranded molecule whereas RNA is usually a single-stranded molecule.

Deoxyribonucleic Acid (DNA)
[edit | edit source]DNA is a double-helix structure formed by the intertwining of two DNA strands in which the phosphate group is located on the outside of the molecule, whereas the nitrogenous bases are inside the structure and are connected via hydrogen bonds and stabilized by Van der Waals forces, hydrophobic effects, and charge-charge repulsion. DNA binds metal ions to the phosphate groups and the electron donor groups in the nitrogenous bases. By binding to the negatively charged phosphate groups, the metal ions neutralize the negative charges on DNA, thereby stabilizing the double-helical structure of the DNA. High concentrations of metal ions can be deleterious. If there is a high metal ion concentration, then less stabilization is required by hydrogen bonding between the nitrogenous bases which can lead to mis-pairing of bases and errors during transcription.
Ribonucleic Acid (RNA)
[edit | edit source]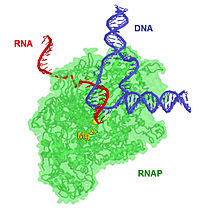
Metal ions bind not only with DNA, but also with RNA. Metal ions interact with the phosphate groups and the electron donor groups in the RNA molecule. This interaction is important because it gives structural stability to the RNA. In addition to this contribution, metal ions interact with ribozymes. By nature, RNA is compact, stable, and folded. They are made of:
1)Ribosomal RNA (rRNA) --- their duty is to catalyze and regulate protein synthesis. Also, it carries the peptidyl-transferase activity and it is part of the structure of the ribosome. 2)Small nuclear RNAs molecules --- involved in the nucleus. It is splicing. 3)Signal recognition particle --- transfers the proteins through the membranes of the cell. Basically, it is a protein target.
In all of these RNAs, metal ions play an essential role in the RNA's structure, formation and catalytic mechanisms. These information are explained in more detail as follow: The pathway for the way that the RNA folds is as follows that: First, the RNA changes from a coil to a secondary structure. The second step is the progression to the tertiary structure. In tertiary structures, the long-range interactions determine the tertiary structure of the nucleic acid.
Because of the phosphate sugar backbone, metal ions play a heavy part in the interactions that RNA goes through to fold and function. Different metal ions with different charges play different charges in various roles of the transformation. Ions with a +1 charge have a part in the charge-screening. This allows the RNA molecules transform to the secondary structure. The tertiary structures of the RNA molecules are stabilized, not with monovalent ions, but with metal ions with two charges.
Holistically speaking, the formation of RNA's tertiary structure is contingent on four different criteria: (a) the RNA sequence, (b) metal ion identity, (c) metal ion concentration, and (d) the presence of RNA binding proteins. The preferred metal is Mg2+ because Mg2+ it is not only helps with stabilizing the tertiary structures, but it also helps the RNA bind to sites of high affinity. However, other metals such as K+, Ca2+, Mn2+, Cd2+, Na+ and Li+ will also suffice. However, their range of reactivity only works for certain RNAs. However, they are vital for RNA metabolism. Trivalent ions besides organic protonated ions are not used.
There are several types of metal ion binding to RNA known:
(a) Diffuse Binding -- performed and done by cations. They are vital to make secondary and tertiary structures.
(b) Site-bound outer-sphere binding of magnesium hexahydrate ion: Water ligands bridge the metal ions and the coordinating atoms on the RNA nucleobase or backbone.
(c) Site-bound inner-sphere binding of Mg2+ to the RNA -- The inner-sphere bound metal ions such as Mg2+ are involved in RNA formation and function.
Proteins
[edit | edit source]protein Proteins are molecules that perform many important functions of the cell that keep it alive. Inorganic molecules and ions are a key part of many of these proteins and their interactions with other molecules. Metal ions can influence both the folding processes and the final structures of many important proteins.
Examples
[edit | edit source]Hemoglobin and Myoglobin
[edit | edit source]
Hemoglobin[[|]] and Myoglobin [[|]]are both oxygen-transport proteins that use metal ions to help carry out their functions. After their polypeptide chains have been sequenced, they bind with Fe2+ ions to form their final structures.[1][2]
Sodium Potassium Ion Pump
[edit | edit source]The Sodium Potassium Ion Pump is used to pump sodium ions out of a cell while at the same time pumping potassium ions in. This function can regulate a number of important cell properties, including the size of the cell and the amount of positive charge inside the cell relative to the outside (known as the resting potential).[3]
Chlorophyll
[edit | edit source]Chlorophyll is the pigment in many plants that gives them their green color and allows them to create their food from sunlight. Chlorophyll uses a Mg2+ ion to start the light reactions of photosynthesis. Chlorophyll a and chlorophyll b are the two different types of chlorophyll that are found in plants. Their structure consists of a porphyrin ring with a central magnesium ion and a long hydrophobic side chain. The difference in the side chain allows chlorophyll to absorb light at different wavelengths. The hydrocarbon tail that is attached to the porphyrin ring makes chlorophyll fat-soluble and insoluble in water.[4]
More Proteins that contain metals
[edit | edit source]Fe (heme): peroxidase, catalase, cytochrome P450, cytochrome c
Fe (without heme): ferredoxin, hemerythrin

Cu: tyrosinase, nitrite reductase, amine oxidase
ZnII: carbonic anhydrase, carboxypeptidase, DNA polymerase
MgII: DNA polymerase
For more information ---> Miessler and Tarr. Inorganic Chemistry. 3rd ed. Pearson Prentice Hall: 2004.
Carbohydrates
[edit | edit source]Carbohydrates form complexes with many metal ions. The hydroxyl group carries a slight negative charge which is increased if the hydrogen atom is deprotonated. This allows carbohydrates to attach to metal ions which carry positive charges through ionic interactions[[|]]. [5]
Inorganic ions can also oxidize or reduce carbohydrates, determining their reactivity. Carbohydrates can be placed in a cupric ion solution. The ones that react can exist as a ketone or an aldehyde and called reducing sugars. These sugars react readily with many molecules. Those that do not react are non-reducing sugars. [6]
Example
[edit | edit source]Calcium
[edit | edit source]In biological systems, carbohydrates have been observed with calcium. Studies show that in an aqueous solution calcium binds to ionic or uncharged carbohydrates. While there is no hard evidence, many believe that carbohydrates may have a role in transporting calcium, calcification, or storage of calcium.
Lipids
[edit | edit source]Lipids are hydrophobic molecules which in the case of fats and oils have as building blocks fatty acid and triglycerides. Other lipids are steroids.waxes. Phosphorylated lipids are the major component of plasma membrane.Link label Page text.[7]
Inorganic compounds such as PO43- and NaOH play an important role in understanding reactions involving macromolecules and their uses.
Examples
1)phosphate in phospholipids.
Phosphates play a major role in the formation of the lipid bilayer of the plasma membrane. Attaching to fatty acid,they form the hydrophilic end of the bilayer whereas the lipid part form the hydrophobic part.
2)Formation of soap: a mixture of sodium salts of different fatty acids. If the fatty acid salt has potassium rather than sodium, a softer lather is the result.
Soap is produced by a saponification or basic hydrolysis reaction of a fat or oil. Currently, sodium carbonate or sodium hydroxide is used to neutralize the fatty acid and convert it to salt.
General overall hydrolysis reaction:
fat + NaOH ---> glycerol + sodium salt of fatty acid
[8] http://www.elmhurst.edu/~chm/vchembook/images/554hydrolysistrigly.gif
References
[edit | edit source]- ↑ [1], Wikipedia-Hemoglobin
- ↑ [2], Wikipedia-Myoglobin
- ↑ [3], Wikipedia-Sodium Potassium Ion Pump
- ↑ [4], Wikipedia-Photosynthesis
- ↑ http://www.transgenomic.com/pd/Chrom/CarbohydrateAnalysis.asp
- ↑ Interactions of metal ions with nucleotides, nucleic acids, and their constituents: Volume 32 of Metal ions in biological systems, Helmut Sigel
- ↑ Link text, additional text.
- ↑ link:http://www.elmhurst.edu/~chm/vchembook/554soap.html