Structural Biochemistry/Nucleic Acid/Nitrogenous Bases/Purines/Thymine
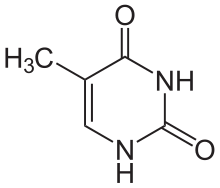
Thymine
[edit | edit source]Thymine is one of the five bases that form nucleic acids, along with adenine, guanine, cytosine, and uracil. The formula of thymine is C5H6N2O2. Thymine is always paired up with adenine through two hydrogen bonds only in DNA to stabilize the nucleic acid structure. Thymine is not present in RNA. Instead, uracil takes place of thymine and binds with adenine. It is a derivative of pyrimidine and can be derived by methylation of uracil at the 5th carbon, hence the other name of thymine, 5-methyluracil. Uracil takes its place in RNA, which also binds to adenine. Thymine is a single ring planar molecule. Thymine combined with deoxyribose yields deoxythymidine while Thymine with ribose makes thymidine.
Thymine binds with deoxyribose to form the nucleoside deoxythymidine, which is the same thing as thymidine. This compound can be phosphorylated with one, two, or three phosphoric acid groups creating thymidine mono-, di-, or triphosphate, respectively.

Thymine is a part of one of the most common mutations of DNA, which involves two adjacent thymines or cytosines. In the presence of UV light, this may form thymine dimers, causing "kinks" in the DNA molecule, interfering with normal function.
Uses of thymine include cancer treatment where it serves as a target for actions of 5-fluorouracil (5-FU). Substitution of this compound to thymine (in DNA) and uracil (in RNA) allows inhibition of DNA synthesis in actively-dividing cells.
Tautaumerization
[edit | edit source]Thymine may go through tautaumerization, interchanging from the keto to the enol functionality by intermolecular proton transfer.
