Structural Biochemistry/Nucleic Acid/Nitrogenous Bases/Purines/Adenine
Adenine
[edit | edit source]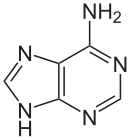
Structure & Function
[edit | edit source]Adenine(A) is one of the four bases that make up nucleic acids. It is a purine base that complementarily binds to Thymine (T) in DNA and Uracil (U) in RNA. This bond is formed by two hydrogen bonds, which help stabilize the nucleic acid structures. Different structures of adenine mainly result from tautomerization of adenine, which allows the molecule to be available in isomeric forms in chemical equilibrium. The molecular formula of adenine is C5H5N5 .
An adenine molecule bound to a deoxyribose, a sugar, is known as deoxyadenosine. An adenine bound to ribose, also a sugar, is known as adenosine, a key component in Adenosine Triphosphate. When adenosine attaches to three phosphate groups, a nucleotide, adenosine triphosphate (ATP) is formed. Adenosine triphosphate is an important source of energy that is used in many cellular mechanisms, primarily in the transfer of energy in chemical reactions. The phosphate of ATP can detach, resulting in a release of energy.
In addition to ATP, adenosine also plays a key role in other organic molecules nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD), both molecules of which are involved in metabolism. Also, adenine can be found in tea, vitamin B12, and several other coenzymes.
Formation and other forms of Adenine
[edit | edit source]In the human body, adenine is synthesized in the liver. Biological systems tend to preserve energy, so usually adenine is achieved through the diet, the body degrading nucleic acid chains to obtain individual bases and reconstructing them through mitosis. The vitamin folic acid is important for adenine synthesis.
Adenine forms adenosine, a nucleoside, when attached to ribose, and deoxyadenosine when attached todeoxyribose; it forms adenosine triphosphate (ATP), a nucleotide, when three phosphate groups are added to adenosine. Adenosine triphosphate is used in cellular metabolism as one of the basic methods of transferring chemical energy between reactions.
In older literature, adenine was sometimes called Vitamin B4. However it is no longer considered a true vitamin (see Vitamin B). Some think that, at the origin of life on Earth, the first adenine was formed by the polymerizing of 5 hydrogen cyanide (HCN) molecules.
Biosynthesis
[edit | edit source]Adenine is one of the byproducts of the Purine metabolism, where inosine monophosphate (IMP) is synthesized with a pre-existing ribose through a complex process involving atoms from the amino acids glycine, glutamine, and aspartic acid, in addition to the formate ions transferred from coenzyme tetrahydrofolate.
]== Tautomerization ==
Tautomers are isomers related by changing the positions of attachment of a single hydrogen and a single double bond, in a three-atom system, such as the keto- and enol tautomers of a ketone. Like, keto-enol tautomers, Adenine, as well as Cytosine, Guanine, Tyrosine, and Uracil may go through tautomerization, interchanging from the amino to the imino functionality by intermolecular proton transfer.
 File:Http://i.imgur.com/l0lfQ.gif
Uracil
File:Http://i.imgur.com/yTNRT.gif
Cystein
File:Http://i.imgur.com/1hwJR.gif
Guanine
File:Http://i.imgur.com/WGmXH.gif
Thymine
File:Http://i.imgur.com/l0lfQ.gif
Uracil
File:Http://i.imgur.com/yTNRT.gif
Cystein
File:Http://i.imgur.com/1hwJR.gif
Guanine
File:Http://i.imgur.com/WGmXH.gif
Thymine
Reference
[edit | edit source]http://www.highbeam.com/doc/1G1-233827562.html http://en.wikibooks.org/wiki/Structural_Biochemistry/Nucleic_Acid/Sugars/Deoxyribose_Sugar http://en.wikibooks.org/wiki/Structural_Biochemistry/Nucleic_Acid/Sugars/Ribose http://en.wikibooks.org/wiki/Structural_Biochemistry/Nucleic_Acid/Phosphate