Structural Biochemistry/Nucleic Acid/Nitrogenous Bases/Purines/Cytosine

Cytosine
[edit | edit source]Cytosine is part of the pyrimidine family, and it is one of the 5 nucleotide bases found in both DNA and RNA. The molecular formula of cytosine is C4H5N3O. Cytosine consists of a heterocyclic aromatic ring, an amine group at C4, and a keto group at C2. Cytosine binds with ribose to form the nucleoside cytidine and with deoxyribose to form deoxycytidine.


The molecule is of planar geometry and cytosine forms 3 hydrogen bonds with Guanine in the DNA double helix. The nucleoside of cytosine is cytidine in RNA, which consists of cytosine and ribose. In DNA, it is called deoxycytidine, which consists of cytosine and deoxyribose. The nucleotide of cytosine in DNA is deoxycytidylate which consists of a cytosine, ribose and phosphate.
Properties
[edit | edit source]Cytosine is a pyrimidine derivative, with a heterocyclic, aromatic ring, and two substituents devoted. Heterocyclic compounds are organic compounds (those containing carbon) that have a ring structure containing atoms with carbon like sulfur, oxygen, or nitrogen as part of the ring. Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. In organic chemistry, a substituent is an atom or group of atoms replaced in place of a hydrogen atom on the parent chain of a hydrocarbon.
In DNA and RNA, cytosine is paired with guanine. However, it is integrally not stable, and can alter into uracil. This can lead to a point mutation if not restored by the DNA repairenzymes, such as uracil glycosylase, which cleaves a uracil in DNA.
Cytosine can also be methylated into 5-methylcytosine by an enzyme called DNA methyltransferase.
History
[edit | edit source]In 1894, Cytosine was discovered by the hydrolysis of the calf thymus tissue. The first structure for cytosine was published in 1903 and the structure was validated when it was synthesized that same year.(The Columbia Encyclopedia)
Chemical Activity
[edit | edit source]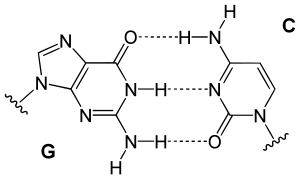
From the image on the left, it can be seen that Guanine and Cytosine bond together through noncovalent hydrogen bonding at three distinct sites. An interesting note is that Watson and Crick first hypothesized that Guanine and Cytosine bonded together through hydrogen bonding at two distinct sites. [1]
Cytosine is found in DNA and RNA or as a part of a nucleotide. When the nucleoside cytidine binds with three phosphate groups, it forms cytidine triphosphate (CTP). This molecule can act as a co-factor to enzymes and it aids in transferring a phosphate to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP) to prepare the ATP to be used in chemical reaction.
In DNA and RNA, cytosine binds with guanine through 3 hydrogen bonds. However, this unit is unstable and can change into uracil. This process is called spontaneous deamination. This can possibly lead to a point mutation if DNA repair enzymes such as uracil glycosylase does not repair it by cleaving uracil in DNA.
Tautomerization
[edit | edit source]Cytosine may go through tautomerization, interchanging from the amino to the imino functionality by intermolecular proton transfer.

References
[edit | edit source]- ↑ Crick, Francis H. (1953). "Molecular Structure of Nucleic Acids". Nature. 171: pp. 737-738.
{{cite journal}}:|pages=has extra text (help); Unknown parameter|month=ignored (help)
Berg, Jeremy M. John L. Tymoczko. Lubert Stryer. Biochemistry Sixth Edition. New York: W.H. Freeman and Company, 2007.
CYTOSINE. The Columbia Encyclopedia, Sixth Edition
