Structural Biochemistry/Nucleic Acid/Nitrogenous Bases/Pyrimidines
What is a Pyrimidine?
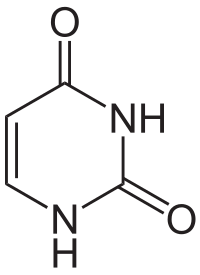
[edit | edit source]A pyrimidine is a 6-membered heterocyclic organic compound made up of 4 carbon atoms and 2 nitrogen atoms at positions 1 and 3.[1] It is one of three isomers of diazine, the other two being pyridazine (1,2-diazine), and pyrazine (1,4-diazine).[2] Pyrimidines are aromatic and planar. The nucleobases Cytosine(C), Uracil(U), and Thymine(T) are all examples of pyrimidines; each with different chemical groups. Pyrimidines can attach to a phosphate sugar group such as a ribonucleotide(which have a hydroxy group positioned axially at carbon-2) or deoxyribonucleotide(which have a hydrogen atom at C-2) through a glycosidic linkage at the 1st Nitrogen to form a nucleotide, the monomeric building block of nucleic acids (DNA and RNA).

Correct mistake:
2. It needs carbonyl phosphate synthetase, which is located in the cytoplasm.
Pyrimidine Biosynthesis
[edit | edit source]1. Unlike in purine, the ring is synthesized first then conjugated after.
2. It needs carbamoyl phosphate synthetase, which is located in the cytoplasm.
3. It also needs an enzyme in order for the reaction to work, but the enzyme should be controlled in 2 steps:
- controlled level at where the reaction occurs & transcriptions must be reduced
- the pyrimidine nucleotides which produces the feedback inhibition level also must be controlled
4. The ring then closes.
5. The C-C bond is formed when the ring oxidizes.


Chemical Properties
[edit | edit source]Pyrimidine has similar properties to that of pyridines. One similarity is that as the number of nitrogen atoms in the ring increase, the ring pi electrons become less energetic and, as a result, electrophilic aromatic substitution gets more difficult while nucleophilic aromatic substitution gets easier. One example is the displacement of the amino group in 2-aminopyrimidine by chlorine and its reverse reaction. Reduction in resonance stabilization of pyrimidines leads to the addition and ring cleavage reactions, and not substitutions. An example of this is in the Dimroth arrangement. Pyrimidines are less basic than pyridines and the N-alkylation and N-oxidation are more difficult in pyrimidines as well.