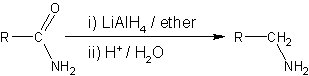Structural Biochemistry/Organic Chemistry/Organic Functional Group/Amino
Introduction
[edit | edit source]
Amino groups are composed of a N atom bonded to two H atoms. Amino groups can act as a base because they can pick up an H+ from a solution. Amino groups can be ionized with a 1+ charge under basic condition. Organic compounds with an amino group are called "amines"; organic compound containing an amino group and carboxyl group are called amino acids which are the building blocks of proteins.
Amines can readily form hydrogen bonds. The amines in the bases of DNA form hydrogen bonds with nearby nitrogen or oxygen atoms and keep the two strands together.
The amino group of lysine[[|]], for example, has be proven useful in the regulation of genes. In particular when the amino group of Lysine in the histones is acetylated, it can no longer function as it regularly does. This is a regulating step that is involved with gene expression and replication.
Another role amino groups play in biochemistry is in enzymes. In the case of a protease, an enzyme which cleaves amino acids, a tetrahedral transition state is formed when the hydroxy group of serine attacks the carbonyl carbon of the amino acid. Because the tetrahedral transition state has a negative charge, the positive NH3+ charges help to stabilize the transition state, forming what is called an oxyanion ring.
Amines can also act as nucleophiles because of its lone pair of electrons. This is the basis by which peptide bonds are formed, with the carbonyl carbon acting as the electrophile in a dehydration reaction.
Structure and Physical Properties
[edit | edit source]
The amine nitrogen is sp3-hybridized and tetrahedral shape. The nitrogen non-bonded pair acts like a substituent—the geometry is tetrahedral around nitrogen and the bond angles are all around 109°. Unlike carbon, sp3 hybridized nitrogen is not rigid and undergoes rapid inversion at room temperature. It is similar to an umbrella flipping inside-out and similar to the inversion of configuration which occurs in an Sn2 reaction.
Nomenclature
[edit | edit source]All the usual IUPAC rules are followed. The suffix –amine is added to the name at the end and the position of the amino group must be specified. For 2° and 3° amines, the largest alkyl substituent is chosen as the parent and the other alkyl groups on nitrogen are named as substituents with the prefix N- to denote that they are attached to nitrogen. Because the amine functional group has the lowest priority in naming, it is often named as a substituent on more highly functionalized molecules.
Acidity of Amines
[edit | edit source]Amines are much less acidic than alcohols with a pKa ~ 35 and Keq = 10-35. A very strong base like alkyllithium must be used to completely deprotonate an amine.
Basicity of Amines
[edit | edit source]Amines are the most basic of the common organic functional groups, but are still fairly weak bases. Protonation occurs on the non-bonded electron pair exclusively. The basicity of amines is directly dependent on the “electron density” at the nitrogen atom. Both inductive and resonance effects can alter the basicity of a nitrogen atom.
Hybridization on the N also affects basicity. An increase in s character on an atom increases the electronegativity of that atom which favors acidity and therefore disfavors basicity. Hence sp3-hybridized nitrogen is more basic than either sp2 or sp hybridized nitrogen.
Synthesis of Amines
[edit | edit source]
1. Cyanide displacement of alkyl halides followed by reduction
2. LiAlH4 reduction of amides
3. Displacements with azide followed by reduction
4. Gabriel synthesis to 1° amines
5. Reductive amination
IR Spectroscopy for Amines
[edit | edit source]Primary Amines will give two short, sharp equal peaks at around 3200 cm-1 – 3500 cm-1
Secondary Amines will give one short, sharp peak at around 3320 cm-1
Tertiary amines will not give a peak in any region.
Reference
[edit | edit source]1. http://orgchem.colorado.edu/Spectroscopy/irtutor/aminesir.html