Structural Biochemistry/Organic Chemistry/Organic Functional Group/Carbonyl
General Information
[edit | edit source]

A carbonyl group is a functional group that is comprised of a carbon atom doubly bonded to an oxygen atom. The carbon attached to the oxygen can have single bonds to different atoms. The atoms that the carbon is bound to distinguish it as a ketone, aldehyde, carboxylic acid, ester, or amide.
[1]==The Nomenclature of Carbonyl Compounds==
1, Naming Carboxylic Acids:
The functional carboxylic acid is called a carboxyl group. In systematic nomenclature, a carboxylic acid is named by replacing the last alphabet "e" with "oic acid." For instance, a two carbon alkane is called ethane and two carboxylic acid is ethanoic acid. Notice that a carboxylic acid with 6 or less carbons are usually known by their common names. ( methanoic acid = formic acid, ethanoic acid = acetic acid, propanoic acid= propionic acid, butanoic acid= butyric acid, pentanoic acid= valeric acid, hexanoic acid= caproic acid)
2, Naming acyl halides
Acyl halides have a halide in place of the -OH group in a carboxylic acid. Acyl halides are named by replacing "-ic acid" in the acid name with "-yl halide" (eg; -yl chloride, -yl bromide, -yl flouride etc). If acid has a name with "-carboxylic acid," then replace "carboxylic acid with "-carbonyl halide" (e.g. -carbonyl chloride, -carbonyl bromide, -carbonyl fluoride)
3, Acid anhydrides
An acid anhydride is formed by two molecules of carboxylic acid reacting with each other to lose one water molecule. An anhydride is a symmetrical anhydride if the two reacting acid molecules are the same. If two reacting acid molecules are different then they are going to from a mixed anhydride. Symmetrical anhydrides are named by replacing "acid" in acid name with "anhydride." Mixed anhydrides are named by stating the names of both acids in alphabetical order then followed by "anhydride."
4, Naming Esters
If there is an -OR group in place of the -OH group of a carboxylic acid, the name of the group attached to the carboxyl oxygen should be
stated first, then state the name of acid by replacing "-ic acid" with "-ate."
Salts of carboxylic acids are named in the same way. The cation is named first then name the acid with replacing "-ic acid" by "-ate" Notice that cyclic esters are called lactones. Their common names are derived from the common name of the carboxylic acid, which designates the length of the carbon chain, and a Greek letter to indicate the carbon to which the carboxyl oxygen is attached.
5, Naming Amides
An amide has an -NH2, -NHR, or -NR2 group in place of -OH group of a carboxylic acid. They are named by replacing "-oic acid", "-ic acid", or "-ylic acid" of the acid name with "-amide." If a substituent is bonded to the nitrogen, the name of the substituent should be stated first (if there is more than one substituent bonded to the nitrogen, they should be stated alphabetically), then state the name of the amide. The name of each substituent is preceded by capital N to indicate that the substituent is bonded to a nitrogen. Cyclic amides are called lactams. Their nomenclature is similar to that of lactones.
Carbonyl Compounds
[edit | edit source]| Compound | Aldehyde | Ketone | Carboxylic Acid | Ester | Amide |
| Structure | 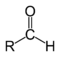 |
 |

|
Each of the characteristic specific to each compound is detailed on their specific pages.
General Reactivity
[edit | edit source]Oxygen is far more electronegative than carbon, so the electron density is higher near the oxygen and lower near the carbon. This creates a dipole moment, where the oxygen bears a negative charge and the carbon bears a positive charge of the same magnitude. This distribution of charges makes carbon an electrophile and oxygen, the resulting nucleophile.
The reactivity of each carbonyl compound is dependent on the group attached directly onto the alpha carbon. This is because of the resonance stabilized structures which form due to the donation of an electron by this group. The stronger the contribution of this resonance structure, the stronger the stability of the carbonyl. An example of this can be seen in carboxylic acids which upon deprotonation create a degenerate structure and thus increases the acidity of the proton. Peptide bonds are unusually strong and carry more of an $sp^2$, planar hybridization due to the resonance contribution of the amide electron onto the alpha carbon. This decreases the reactivity of peptide bonds considerably and thus requires much energy or a protease to catalyze the bond. Carbonyls by themselves are very stable bonds and the energy of their formation are usually very high. This makes the formation of carbonyls in organic synthesis to be highly thermodynamically favorable and usually the creation of a carbonyl bond as the end product will drive a reaction to formation.
The carbonyl group has a short, strong, and very polar double bond. Its reactivity of its double bond is very different from the double bond of the alkenes because of their oxygen's electronegativity along with the lone pair of electrons. The carbonyl carbon is also electron withdrawing since it is so close to the highly electronegative oxygen. The polarization of aldehydes and ketones also alters the physical constants. The polarization of the carbonyl group is the reason why their boiling points are higher than those of the hydrocarbons of similar molecular weight. the carbonyl group's polarity causes the smaller molecules to be completely miscible in water. Carbonyl compounds with more than six carbons are considered large, which is insoluble in solution. The larger the compound, the larger its hydrocarbon chain, the more hydrophobic the molecule is, so its solubility would then decrease.
Inductive Effect Take important notice that the electrophilicity of the carbon is highly dependent on the nearby atoms and the atoms it is directly bounded to. For example, the carbonyl carbon of a carboxylic acid will not be as electrophilic as a carbonyl carbon of a ketone because of resonance stabilization. On the other hand, acetyl chloride (the carbon is bound to an R-group, doubly bonded to the oxygen, and then bound to the fluorine), will have a more electrophilic carbonyl carbon because the electronegative chlorine will increase the induced positive dipole of the carbon. Electrophilicity plays a key role in chemical reactions, and less electrophilic carbonyl carbons are not as readily reactive.
Biochemical Synthesis of Carbonyl Groups
[edit | edit source]
Within biological systems, carbonyl compounds can be formed by the oxidation of alcohols. An oxidation reaction in organic chemistry is one that is characterized by a process which either adds electronegative atoms or removes hydrogen from a molecule. An example of this oxidation includes the oxidation of ethanol by the oxidizing agent nicotinamide adenine dinucleotide (NAD+). NAD+ is composed of a pyridine ring, two ribose molecules, and the heterocycle adenine. When the two enantiometers of 1-Deuterioethanol are reacted with NAD+, in the presence of enzyme alcohol dehydrogenase, the biochemical oxidation is found to be stereospecific (NAD+ and only removes the hydrogen attached to the C1 atom in 1-Deuterioethanol. [2] Similar to the oxidation of 1-Deuterioethanol described above, other alcohols can be oxidized biochemically to form carbonyl groups. Another example includes the oxidation of methanol to formaldehyde. [2]
'HNMR
[edit | edit source]In 'HNMR spectroscopy, aldehyde compounds have a very unique chemical shift which appears to be between 9-10 ppm. Which means that the aldehyde formyl hydrogen is also very strongly deshielded. Aldehyde C2 hydrogens are also slightly deshielded because of the electron withdrawing from the oxygen from the carbonyl group. In ketones, this is also similar. The alpha-hydrogens also experience this deshielding; which has a chemical shift between 2-2.8 ppm.
13CNMR
[edit | edit source]The carbon-13 NMR spectra for aldehydes and ketones are the same since it is of the chemical shift of the carbon participating in carbonyl group. Since that carbon is bound to an oxygen, it will appear at a lower field approximately 200 ppm. The carbons adjacent to the carbonyl carbon are also deshielded (just like the hydrogens in 'HNMR). The carbons further away from the carbonyl group are less deshielded.
IR Spectroscopy
[edit | edit source]The IR absorbtion bands for all carbonyl compounds absorb in the 1760-1665 cm-1 region, which is due to the streching vibration of the C=O bond. Generally, carbonyl groups have a high intensity and narrow regions, making them useful for diagnostic purposes.
| Range | Type of Compound | Such as: |
|---|---|---|
| 1750-1735 cm-1 | saturated aliphatic esters | CH3-CH2-COOR |
| 1740-1720 cm-1 | saturated aliphatic aldehydes | CH3-CH2-COH |
| 1730-1715 cm-1 | α, β-unsaturated esters | R'(R")C-CH-COOR |
| 1715 cm-1 | saturated aliphatic ketones | CH3-CH2-CO-CH2-CH3 |
| 1710-1665 cm-1 | α, β-unsaturated aldehydes and ketones | R'(R")C-CH-COH |
- ↑ Bruice,Paula Yurkanis.Organic Chemistry Six edition, Pearson Education,Inc. New York. 2010
- ↑ a b Invalid
<ref>tag; no text was provided for refs namedSchore - ↑ "IR: Carbonyl Compounds." IR: Carbonyl Compounds. TurnKey Linux, 2008. Web. 20 Nov. 2012. <http://orgchem.colorado.edu/Spectroscopy/irtutor/carbonylsir.html>.
