Structural Biochemistry/Chemistry
Chemistry is the study of composition, structure and properties of matter. This includes studying changes that are observed when undergoing various chemical reactions or understanding the basic structure of complex macromolecules. It provides an understanding for atoms, molecules, crystals which make up everything in this world, while incorporating the concepts of energy and entropy.
Chemical Foundations of Biochemistry
[edit | edit source]Chemistry is always interconnected with the other sciences, such as astronomy, physics, material science, and biology. In terms of biochemistry, it strives to find chemical explanations to biological form and function. Chemistry shows that all forms of life share common origins through the similarity of various chemical pathways between different organisms. For example, all living cells have the common molecules of amino acids, carbohydrates, lipids, and nucleic acids which all perform the same functions in every cell.
Nature is predominantly made of four elements: Hydrogen (H), Oxygen (O), Nitrogen (N), and Carbon (C). Together they make up over 99% of all living cells. These elements are the lightest atoms that are capable of making up to four stable and strong bonds. There are other elements that are also essential to living organisms such as Sodium (Na), Potassium (K), Calcium (Ca), and Sulfur (S). These are called trace elements and usually are needed to help specific proteins to function.[1]
Organic Chemistry as the Backbone of Biochemistry
[edit | edit source]Organic chemistry is the study of the structures, properties, synthesis, and reactions of chemical compounds consisting of mainly carbon and hydrogen atoms. It is the backbone science that explains many of the interactions that occur in a cell at the most basic level. The majority of biochemistry, from a chemical point of view, involves the interactions between organic molecules that exist in organisms and how they are utilized in a versatile fashion at a cellular level. The arrangement of bonds within a molecule determines its overall shape, conformation, and the molecules ability to perform specific functions within a cell. Organic compounds that are of particular interest in biochemistry are carbohydrates, nucleic acids, and proteins.
Carbon is unique from the other essential elements for it has the capability to form four stable, covalent bonds and stable double and triple bonds. Carbon can bond with other carbons to create long chains with or without branches or cyclic structures allowing the formation of an endless array of molecules with different shapes, sizes, and compositions. Replacing hydrogen with other elements or functional groups gives the organic molecule different properties such as polarity.
Importance of Chemical Structure
[edit | edit source]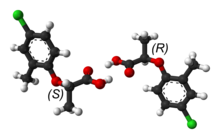
Biochemistry chemistry is stereospecific meaning that the 3-D orientation of a molecules matters and only specific arrangements will interact properly. Stereoisomers are molecules that have the same chemical bonds but have a different spatial arrangement. They may look very similar but they cannot be the same molecule with out breaking any of the covalent bonds.

There are many places where there can be varying spatial configuration. A molecule can have a chiral center which means that an atom (usually carbon) that is attached to four different substituents. These four can arrange themselves any way they want to around the carbon but if the arrangements are not exactly the same then they are stereoisomers. One example is if the molecule is a mirror image of itself. No matter how you rotate the molecule it will never superimpose on to its original form. This specific type of stereospecificity is called enantiomers. If they are not mirror images of each other they are called diastereomers.
Another way is to have a double bond. Double bonds, unlike single bonds, are stiff and do not rotate freely. The four substituents that surround the bond can form either trans, where the two highest ranked substituents are opposite sides of the bond, or cis, where the highest ranked are on the same side of the bond, relationships. This is called either geometric isomers or cis-trans isomers.
While enantiomers have almost the same chemical properties only the specific orientation will have the desired effect in a biological system. In nature, chiral compounds are usually found in only one orientation. For example, in carbohydrates the second to last carbon always has a "D" orientation and amino acid's chiral carbon is always in an "L" orientation. Enzymes can discriminate between enantiomers therefore is essential to have the right configuration.
Water and its Effects on Structure
[edit | edit source]The field of biochemistry also encompasses the special properties of water. Because all living organisms have cells that interact with aqueous solutions, the properties of water are fundamental to the environment in which biochemical reactions occur. The structure of a molecule determines whether it is hydrophilic or hydrophobic. Its interaction in water determines how these molecules function in a cell, one example being the lipid bi-layer in the cell membrane and how its chemical properties determines its function in the cell (in this case acting as a barrier in a cell). Water also has unique features such as high boiling and melting points. Its liquid form is also more dense than its solid form which allows marine life. An important feature of water in regards to biochemistry is its ability to form hydrogen bonds.
References
[edit | edit source]- ↑ Principles of Biochemistry by Lehninger
