Structural Biochemistry/Water

Water (molecular formula H2O) is an essential part of all living organisms, making up 70% or more of the weight of most organisms. It consists of an oxygen atom connected to two hydrogen atoms by polar covalent bonds. It is considered to be the universal solvent for many reasons including its structural, chemical and physical properties. These properties result in the many unique characteristics of water.
Structure
[edit | edit source]Intramolecular Structure
[edit | edit source]
Water is a compound that consists of two hydrogen atoms and one oxygen atom attached together by two sigma bonds and with two lone pairs of electrons around the oxygen atom. This attachment of the hydrogen nucleus to the central oxygen atom by electrons is called a covalent chemical bond. These components of a water molecule generally form a tetrahedral arrangement around the oxygen atom, having an expected bond angle of 109.5° between each component. However, this is not the case due to the repulsive forces of the lone electron pairs. As a result, the electrons push the hydrogen atoms closer together, resulting in a bond angle between the hydrogen atoms of 104.5°. The geometry of water molecules is typically referred to as "bent" or "angular". Since this is a bent structure and not a linear structure, the distribution of charges is not symmetrical which gives a polar trait to water molecules.
Intermolecular Structure
[edit | edit source]
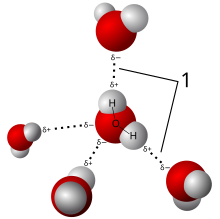
The partial charges on the oxygen and the hydrogen allow for water to participate in hydrogen bonding. The partial negative charge on the oxygen is attracted to the partial positive charge on the hydrogen of another water molecule. The oxygen atom is partially negative because the oxygen nucleus draws away the electrons from the two hydrogen atoms. Thus, the net charge of the hydrogen atoms becomes partially positive. Due to this hydrogen bonding characteristic, the water molecule exhibits an attractive force to other water molecules and has the ability of ionization. Hydrogen bonding, although much weaker than ionic and covalent bonding, is one of the strongest electrostatic interactions, and is responsible for many of water's unique properties, such as high melting and boiling points, high heat of vaporization, and high surface tension.
Chemical and Physical Properties of Water
[edit | edit source]Some of the unique properties of water include high boiling point, high melting point, high heat of vaporization, high surface tension,and high heat capacity. All these properties indicate that inter-molecular forces of attraction between water molecules are high.
Hydrogen Bonding
[edit | edit source]High melting point, boiling point, and heat of vaporization are unique qualities of water caused by the attractions between adjacent water molecules. Each hydrogen of a water molecule shares an electron pair of the central oxygen atom. Because oxygen is more electronegative, its nucleus attracts electrons more strongly than the hydrogen nucleus. The result gives each hydrogen a partial positive charge, and the oxygen a partial negative charge, otherwise known as a dipole moment. This allows for hydrogen bonding, the electrostatic attraction between the hydrogen atom of one water molecule to the oxygen atom of another, as well as resulting in the following properties:
High Heat Capacity
[edit | edit source]Specific heat is a measure of heat required to raise the temperature of 1 gram of water 1°C. Water has a high heat capacity, meaning that it changes temperature slowly after gaining or losing energy. In fact, water has the second highest specific heat capacity of any known substance after ammonia. The heat capacity of water is a property directly resulting from hydrogen bonding. The combined effects from hydrogen bonding are enormous, although hydrogen bonds are linked by weak non-convalent interaction. When heat is absorbed, hydrogen bonds are broken and water molecules can move freely. When the temperature of water decreases, the hydrogen bonds are formed and release a considerable amount of energy. The resistance to sudden temperature changes makes water an excellent habitat, allowing organisms to survive without experiencing wide temperature fluctuation. Furthermore, because many organisms are mainly composed of water, the property of high heat capacity allows highly regulated internal body temperature.
High Melting Point
[edit | edit source]
Water has an unusually high melting point when compared to that of other Group 6A hydrides (Methane, ammonia), due to the hydrogen bonding between water molecules. In its solid form (ice), each water molecule is subject to four different hydrogen bonds - two hydrogens that are capable of each being a hydrogen bond donor and an oxygen that is capable of accepting two hydrogen bonds from neighboring molecules.
High Boiling Point
[edit | edit source]
Just like with its melting point, water molecules also have a higher boiling point than one would expect if the boiling points of other Group 6A hydrides were extrapolated. Likewise, the reason for this is the hydrogen bonding between neighboring water molecules. Because hydrogen bonding is a relatively strong intermolecular force, high heat energy is required to break up the force.
High Heat of Vaporization
[edit | edit source]Heat of vaporization is the quantity of heat required to transform 1 gram of liquid water into its gaseous form. Similarly to the high specific heat of water, the high heat of vaporization is also due the hydrogen bonding. The earth is mostly covered by water, thus water having a higher heat capacity provides a much more stable climate on earth. Evaporative cooling occurs when liquid molecules with the highest kinetic energy escapes as gas, leaving the remaining molecules with lower kinetic energy. This property of water stabilizes temperature in lakes and ponds. It prevents plants and animals from becoming over-heated when exposed to excess heat.
Colligative properties
[edit | edit source]"Colligative properties" are defined as the properties of solutions that are correlated to how many molecules are in a given volume of solvent instead of what the molecules are. Examples of these properties are the decrease of vapor pressure, the increase of boiling point, the decrease of freezing point and the depression of osmotic pressure. They apply to water as well. When a solute, like NaCl, is added into water to form a solution, the boiling point of the whole solution will increase. Surrounded by water molecules, the lattice structure of NaCl will break down due to the displacement of solute-solute interaction by stronger solute-water interaction. Then there are fewer free-moving water molecules in the solution which decrease the entropy of the system. In order to balance that, higher enthalpy change is required to break the intermolecular forces so as to boil the solution to its gas state.
The freezing point is lowered when solute is dissolved in water. The reason for that is because that the size of the solute molecule may not fit into the water crystal structure and less free space is offered for other molecules to move when the molecule of liquid joins the crystal. So higher enthalpy change is required to balance the loss in entropy and replace the solute molecule by water molecules. A common example in our daily life is adding salt to snow in winter in order to melt it at a faster speed. Thus, colligative properties are all determined by the properties of solutes other than by their amount of molecules.
Density Relationships
[edit | edit source]Liquid and Solid Phase Densities
[edit | edit source]Water is one of the few substances that has the exception of having less dense solid than its liquid. Hydrogen bonding is the reason behind ice floating to the top of liquid water. It is because of this special property of water that enables for the survival of life inside a lake during cold weathers because the lake freezes top down rather than bottom up. As the temperature of water drops below 0°C, each water molecules forms a crystalline lattice with four other neighbors. The hydrogen bonds keep each molecule at a distance that makes ice less dense than liquid water. The crystalline structure of ice as the result of hydrogen bonds is disrupted as temperature increases. The hydrogen bonds aggregate and become denser as crystals collapse. Thus, the density of water is highest at 4°C.
Cohesion of Water Molecules
[edit | edit source]High Cohesion
[edit | edit source]High cohesion is a property in which individual water molecules tend to “stick” with other water molecules due to hydrogen bonding. Hydrogen bonds are very fragile in the liquid form of water, and the property of cohesion is observed when hydrogen bonds, collectively, hold water molecules together. These bonds form, break, and re-form at a high frequency, making water molecules more structured than most other liquids. High cohesion leads to two significant characteristics of water: strong capillary action and high surface tension. The cohesion of water molecules is a crucial property in the transportation of water to plants. Strong capillary action is essential to plants in the effective transport of water. Water is willing to diffuse to dry areas especially in a small-spaced fabric, where water will use its cohesion property to attract or pull the water molecules behind before the water molecules evaporate. Therefore, the transportation of water molecules can keep moving even when the plant has a high evaporation rate. Without this property, plants will end up dehydrated and unable to continue photosynthesis
High Surface Tension
[edit | edit source]
Surface tension is a property that stems from cohesion. Water has a higher surface tension than most other liquids except mercury because of the hydrogen bonds between water molecules. The ordered arrangement of water molecules result in a strong interface for surface tension. This property allows water to act as a stretchable film. It also serves as a supporting surface for many aquatic organisms, which have evolved to spread their body weight over a large surface area without breaking the water's surface tension.
Amphoteric
[edit | edit source]Water is known to be amphoteric, which means it is capable of acting as a base or an acid. When the water is a part of a solution, the equilibrium can change. When water reacts with a stronger acid, water will act as a base, and vice versa, water acts as an acid with a stronger base. Due to the oxygen atom’s two lone pairs in the water molecule, water will often act as an electron pair donor, or a Lewis base in reactions involving Lewis acids. However, water can react with Lewis bases, by forming hydrogen bonds between the hydrogen atoms of water and the electron pair donors.
Universal Solvent
[edit | edit source]Water is considered the universal solvent due to its ability to dissolve or dissociate most compounds. This property is due to its polarity, where oxygen has a higher electronegativity than hydrogen. In a solution, the positive hydrogen side of water is attracted to the negative parts of the compound being dissolved, and vice versa with the negative oxygen being attracted to the positive parts. Thus, water can dissociate and break apart ionic compounds.
High Dielectric Constant
[edit | edit source]- Dielectric Constant - Dielectric matter implies that the electric charges are associated with a particular nucleus; these electrons are "bounded" to the atom. The presence of an electric field will induce a net dipole, meaning that electrons will move toward the positive potential but remain bounded to the molecule; the dielectric matter is then said to be polarized. Water has a dipole moment of 6.17×10−30.
The dielectric constant is a measure of the extent of the polarization. Water's high dielectric constant is due to the water molecule having a dipole moment in which water can be polarized. Because of a high dielectric constant, water has the ability to surround ions therefore diminish attractions from other charges, making it a good solvent for ionic or polar substances, hence its name as the universal solvent.
Solvency on Polar Substances
[edit | edit source]Due to the polar nature and how readily water molecules can undergo hydrogen bonding, uncharged but polar molecules can easily be dissolved. This is primarily due to the stabilizing effect caused by the hydrogen bonding between a polar molecule and a water molecule. Ketones, alcohols, aldehydes, and compounds that have N-H bonds, form hydrogen bonds with water molecule. These biomolecules tend to be very soluble in water.
Hydrogen bonds between an uncharged polar molecule and water tend to be the strongest when the hydrogen atom is in a straight line between two atoms with electronegative atoms. Due to this, hydrogen bonds are considered to be very highly directional.
Hydrogen bonds are able to adjust to the position around the solutes. This means that the pattern of hydrogen bonds must recognize the difference in shape and size in order to adapt to the new molecule.
Water also forms a hydration shell. This occurs in an aqueous solution when a solute dissolves in the solution. The solute is then surrounded by the partially positive hydrogen atoms and later binds to the solute. This ultimately creates new bonds and makes a solution.
Solvency on Charged Substances
[edit | edit source]Water also has a stabilizing effect on ions such as Na+ and Cl-. By hydrating both ions, the electrostatic interaction between both ions are greatly diminished and it weakens their tendency to form crystalline lattice structures. This also applies to many charged functional groups present in biomolecules such as carboxylic acid, anhydrides, and protonated amines. Water dissolves such compounds with these given types of charged functional groups by replacing solute—solute interactions with solute—water interactions. In doing so, the electrostatic interactions screen between the solute molecules. Water is primarily an effective screening for electrostatic interactions due to its high dielectric constant.
Solvency on Crystalline Substances
[edit | edit source]
Water is a very versatile solvent. When a crystal of the ionic compound, NaCl for example, is placed in water, the sodium and chloride ions are separated in the solvent. The hydrogen atoms of water molecules are positively charged and attract chloride anions. Meanwhile, oxygen atoms of water molecules are negatively charged and attract sodium cations. Water molecules shield individual sodium and chloride ions from one another by hydration; thus, the polarity of water molecules directly affect the dissociation of solutes. Furthermore, when crystalline substances dissolve in water, they acquire greater freedom of motion, which increases the entropy of the system. This resulting increase in entropy of the system is greatly responsible for the ease of dissolving salts in water. In terms of thermodynamics, the formation of the solution occurs with a favorable free-energy change.
Interactions with Nonpolar Compounds
[edit | edit source]


While water acts as a relatively good solvent for polar compounds, this is definitely not the case with non-polar compounds. Not only will non-polar compounds not dissolve in water, but they also cause an unfavorable interaction in water. The non-polar (and usually hydrophobic) portions of a molecule (such as the hydrocarbon backbone of many organic molecules) cause surrounding water molecules to become ordered, which is an unfavorable process since entropy has decreased.
Oftentimes, molecules will also align themselves in a way such that the hydrophilic, polar portions of the molecule will shield the hydrophobic, non-polar portions from surrounding water molecules, forming a micelle. A common example is the "lipid bilayer" in the plasma membrane of a cell, where the polar heads align to shield the non-polar fatty acid tails from water outside of the cell. This way, the hydrophilic heads are exposed to outside water molecules while the hydrophobic tails are almost completely free of water. The micelle structure is important in the absorptions of fat-soluble vitamins and complicated lipids that are found in the human body.
When enzymes and substrates are present in a solution, sometimes ordered water molecules will move away from the middle of the two groups to allow interaction. This phenomenon increases the disorder of molecules, which is an increase in entropy, signifying that the process is thermodynamically favorable. With the water molecules out of the way, the substrate and the enzyme can form an enzyme-substrate complex, which then kicks off the chain of chemical reactions governed by the enzyme. This is an example of Desolvation.
Water as a Reactant
[edit | edit source]Water is not only a solvent for many biomolecules but also plays a huge role in many reactions with biomolecules within living organisms. A prime example is the conversion of ADP to ATP, which is an essential process for storing energy in living organisms. In this process, a condensation reaction occurs which is characterized by the elimination of water when the reactant ADP couples with a 3rd phosphate group; water is thus a product in this reaction. When energy is needed, a type of hydrolysis reaction occurs where water is required to hydrate ATP to release the energy. When water acts as a reactant, such as in a hydrolysis reaction, the electron-rich oxygen will serve as a nucleophile. Hydrolysis reactions tend to be exergonic which means that energy is released. Seeing how two molecules are produced from one molecule due to the aid of water, these types of reactions are favorable since the randomness of the system is increased. Water also tends to an essential component in oxidation reduction reactions that occur in respiration for plants and animals.
Weak Interactions are Crucial to Macromolecular Structure and Function
[edit | edit source]Hydrogen bonds and ionic, hydrophobic, and Van der Waal interactions are much weaker than covalent bonds. Despite their weakness, the cumulative effect of these various interactions can be very significant. For instance, the noncovalent binding of an enzyme to its substrate may involve several hydrogen bonds and at least one or more ionic interactions, as well as some hydrophobic and van der Waals interactions. The formation of each of these weak bonds contributes to the net decrease in the free energy of the system. The stability of the noncovalent interactions can then be measured from the binding energy. Stability varies exponentially with the binding energy. Therefore, macromolecules such as proteins, DNA, and RNA, which have so many sites of potential hydrogen bonding or ionic, van der Waals, or hydrophobic interactions, have many small binding forces that when combined, the force becomes enormous. For macromolecules, the most stable structure is usually that in which weak interactions are maximized. This explains why the folding of a single polypeptide or polynucleotide chain into its three-dimensional shape is determined by this principle.
Reference
[edit | edit source]1.http://intro.chem.okstate.edu/1215/lecture/chapter7/lecture92898.html
2.http://en.wikipedia.org/wiki/Freezing-point_depression
3.http://en.wikipedia.org/wiki/Evolutionary_history_of_plants
4.http://library.thinkquest.org/28751/review/biochem/2.html
5. Lehninger Principles of Biochemistry, Fourth Edition, W. H. Freeman, 2004.
6.http://en.wikibooks.org/wiki/Structural_Biochemistry/Chemical_Bonding/Hydrogen_bonds
