AP Chemistry/The Basics
You should remember everything here from your high-school level chemistry class.
Units and Measurement[edit | edit source]
- Fahrenheit is not used on the AP exam. Celsius (°C) and Kelvin (K) are used. Pure water freezes at 0° Celsius (273K) and boils at 100 °C (373K). Kelvin, on the AP exam, can be converted to Celsius by adding 273.15.
Significant Figures[edit | edit source]
Significant figures are used to ensure that precision is communicated correctly. When measured numbers are given, the last digit is assumed to be ±1. The number 3.5 for example is assumed to range from 3.4 to 3.6 when an exact precision is not given.
- Digits 1 through 9 are significant, and so are zeroes in between them. For example, the number 209 has three significant figures.
- Zeroes to the right of all other digits are only significant if there is a decimal point written. 290 has 2 sig figs, 290. has three, and 290.0 has four.
- Leading zeros are not significant. 0.00209 has only three sig figs.
Measured vs. Exact Numbers[edit | edit source]
Exact numbers are either defined numbers or result from a count. A dozen is defined as 12 objects. A pound is defined as 16 ounces. Exact numbers are assumed to have an infinite number of significant digits. Measured numbers by contrast always have a limited number of significant digits as described previously. A mass reported as 12 grams is implied to be known to the nearest gram and not to the tenth of a gram.
The Mole and Avogadro's Number[edit | edit source]
12 grams of Carbon-12 contain exactly one mole (6.02×1023) of molecules. This is a measured number known as Avogadro's number. It is easy to convert between atomic mass, grams, and particles using Avogadro's number.
Multiplying and Significant Figures[edit | edit source]
Multiplying measured numbers in Chemistry is not like multiplying in math. 5 * 92 equals 460 in math class, but it equals 500 in chemistry. This is because the 5 only has one significant figure, so the answer has to be rounded to one sig fig. If 5.0 and 92 were multiplied, on the other hand, the answer would be 460 in both subjects.
Adding and Significant Figures[edit | edit source]
- First, align all the numbers vertically, as if you were going to add them. DO NOT WRITE IN EXTRA ZEROS AS PLACEHOLDERS.
- Add
- Round to the smallest place that contains a digit in every number.
Example: 210 + 370. + 539
210 370. 539 +---- 1119 ≈ 1120
States of Matter[edit | edit source]
- Solid (s) - definite shape and volume. Vibrates in place, but does not flow.
- Fluids - take the shape of their container.
- Liquid (l) - definite volume
- Gas (g) - variable volume (compressible)
History of Chemistry[edit | edit source]
- Democritus - philosopher who made the idea of atoms.
- Antoine Lavoisier - discovered Law of Conservation of Mass, which states that mass does not appear or disappear in chemical reactions, only rearrange.
- John Dalton - first scientist to scientifically describe atomic theory.
- Matter is made from indestructible particles called atoms.
- Atoms of the same element are the same.
- Compounds are two or more atoms bonded together.
- Chemical reactions are the rearrangement of atoms.
- J.J. Thomson - discovers the electron.
- Robert Millikan - discovers the mass and charge of electrons.
- "Raisin Pudding model" - atoms are like pudding, with electrons as raisins.
- Ernest Rutherford - through his gold foil experiment, discovers the nucleus. Since most of the alpha particles he shot through the gold were not deflected, he concluded that most of an atom is empty space.
Modern Atomic Theory[edit | edit source]
Atoms are made up of protons, neutrons, and electrons. Protons and neutrons weigh approximately 1 AMU, and electrons have a negligible mass. Elements are determined by the number of protons in the atom, known as the atomic number. The number of neutrons varies, creating different isotopes of different mass. The atomic mass of an atom is the sum of its protons and neutrons, both of which are found in the nucleus.
Electrons[edit | edit source]
Electrons are arranged into shells that surround the atom. Each shell has 1-4 subshells, which themselves have 1-7 orbitals, each of which holds two electrons.
| Shell | Subshell | Orbitals |
|---|---|---|
| 1 | s | 1 |
| 2 | s, p | 1 + 3 = 4 |
| 3 | s, p, d | 1 + 3 + 5 = 9 |
| 4 | s, p, d, f | 1 + 3 + 5 + 7 = 16 |
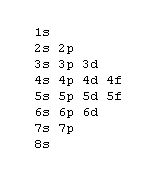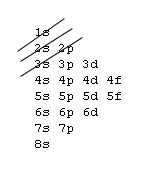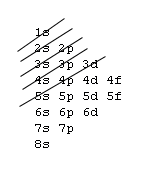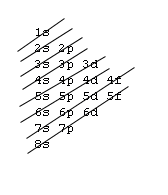
Filling Electron Shells[edit | edit source]
- Aufbau principle - fill the lowest energy subshells first, in accordance with the following image:
- Exception: Elements within the Lanthanide and Actinide series contain nuances and do not strictly follow the gif pattern. Elements on either side of these series do adhere to the gif.
- Hund's rule - fill each orbital in a subshell with one electron before putting a second electron in any of those orbitals.
Writing Electron Configurations[edit | edit source]
E.g. sodium = 1s22s22p63s1
VESPR Theory[edit | edit source]
Valence shell electron pair repulsion (VESPR) theory. Electrons in a compound will try to move as far apart from each other as possible. Bonded pairs repel more strongly than unbonded pairs.
Hybrid Orbitals[edit | edit source]
The moving apart of electron pairs requires the hybridization of orbitals. These hybrids range from sp to sp3d2, having two to six pairs. This leads to several different shapes for each central atom in the molecule.
| ↓Effective Electron pairs & hybridization Lone pairs→ | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| 2 sp | Linear 180° | N/A | N/A | N/A |
| 3 sp2 | trigonal planar 120° | bent | N/A | N/A |
| 4 sp3 | tetrahedral 109.5° | trigonal pyramidal | bent | N/A |
| 5 sp3d | trigonal bi-pyramid 120° 90° 180° | see saw | T-shaped | linear |
| 6 sp3d2 | octahedral 90° 180° | Square pyramidal | Square planar | N/A |
Sigma and Pi Bonds[edit | edit source]
- Sigma bond - forms in all compounds
- Pi bonds - one or more are formed per extra electron pair that is shared among two atoms. These bonds are weaker than sigma bonds.
Resonance[edit | edit source]

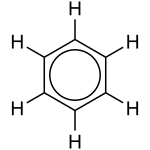
Sometimes, there is more than one "correct" way to draw a substance. In reality, the structure of the substance is an average of the drawn variations.
The Periodic Table[edit | edit source]
You should already be familiar with this. Each row is called a period, and each column is a group or family. Nonmetals and metals are separated by a jagged line on the right side. (Hydrogen is also a non-metal). Elements that border the line are called metalloids, and share characteristics with both metals and nonmetals.
Oxidation Numbers[edit | edit source]
Oxidation numbers are a way of keeping track of electrons and making sure that components of a compound match by the correct ratios.
- Pure elements have an oxidation number of zero.
- Ions, monoatomic or polyatomic have oxidation numbers equal to their charge.
- The sum of the oxidation numbers in covalent and ionic compounds must equal zero.
- Bonded Group 1 metals are +1, Group 2 are +2, and halogens are -1, unless bonded with oxygen.
- Bonded oxygen is -2 unless it is in a hydroxide (OH), where it is -1, or with fluorine, where it is positive.
- Bonded hydrogen is -1 when bonded with a metal and +1 when bonded with a nonmetal
Naming Compounds[edit | edit source]
Binary Compounds[edit | edit source]
(First element's name) (Second element's name + ide) e.g. Sodium Chloride.
Binary Acids[edit | edit source]
Hydro(nonmetal+ic) acid. E.g. Hydrobromic acid (HBr)
Ternary Compounds[edit | edit source]
(First element's name) (polyatomic ion's name) e.g. Sodium Hydroxide (NaOH). Note that there is an exception - the ammonium ion (NH4+) can replace the first element.
Ternary Acids[edit | edit source]
In the following order: (hydrogen)(a nonmetal)(oxygen)
If the ion ends in -ate, the acid will be named (nonmetal)ic acid. Example: H2SO4 contains a sulfate ion. It is called sulfuric acid.
If the ion ends in -ite, the acid is named (nonmetal)ous acid
Stock System[edit | edit source]
Some elements, especially transition metals, can have many oxidation numbers. As a result, the positive oxidation number has to be written in, using Roman numerals. For example, CuO is Copper (II) oxide and Cu2O is Copper (I) oxide.