AP Chemistry/Periodicity
The Periodic Table
[edit | edit source]The periodic table is essentially a summary of a lot of information that is useful as a reference, and will be essential to the AP chemistry test. Here is what it looks like:
Each row is called a period, while each column is called a group. Elements in the same group tend to have similar properties. Make note of the names and locations of each of the groups of elements (alkaline, alkaline earth, etc.) they will be referred to. Each box represents one element. Let's look at one box on the periodic table:

- The small number on the top indicates the atomic number of the element. It also indicates the number of protons in the nucleus of one atom of the element.
- The big two letters in the center indicate the abbreviation of the element. This element is He, or Helium
- The small number at the bottom of the big letters indicate the atomic mass of the element. The atomic mass of an element is the average of the atomic weights of the isotopes of an element, weighted by the abundance of the element (if He-4 occurs 97% of the time and He-5 occurs 3% of the time, then you would do .97 ⋅ 4 + .03 ⋅ 5 and that would be the atomic mass).
Spectrometry
[edit | edit source]Mass Spectrometry
[edit | edit source]The creation of a spectrum based on mass by finding the mass to charge ratio. Used to determine weight of isotopes.
Photoelectron Spectrometry
[edit | edit source]The creation of a spectrum based on the kinetic electrons of an electron when energy is put into an atom and that electron is released.
Trends in the Periodic Table
[edit | edit source]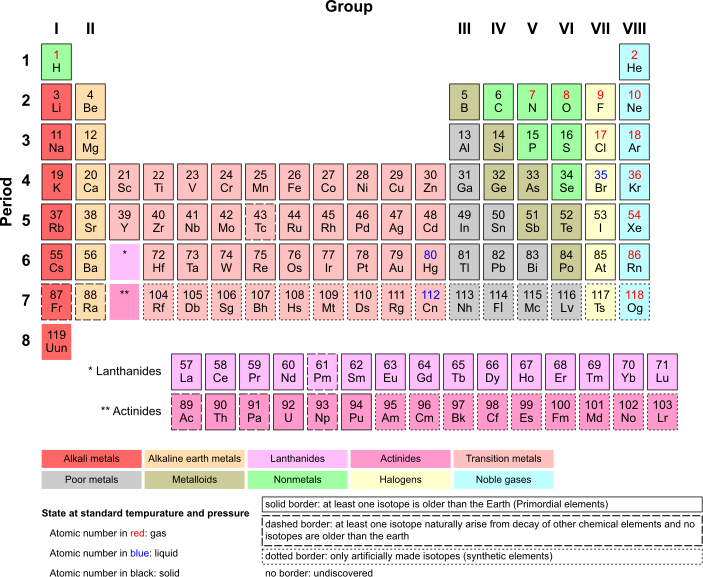
- Metals tend to be more reactive as one goes down and to the left of the periodic table (called metallic character).
- Nonmetals tend to be more reactive as one goes up and to the right of the periodic table (called nonmetallic character).
- Atomic radius increases as one goes from the top to the bottom, and also increases when moving right to left, because effective nuclear charge decreases.
- First ionization energy decreases as one goes from the top to the bottom, and also decreases when moving right to left (opposite of atomic radius). Ionization energy jumps especially high when taking an electron from an element with a full shell.
- Electron affinity (how much an electron wants to accept an electron) Atoms closer to F want electrons (negative affinity value), while elements closer to Fr do not accept electrons (positive affinity value).
- Electronegatvity (the attraction of electrons to individual atoms) has the same trend as electron affinity. It is used to determine how electrons are distributed among atoms in molecules.
- In groups I - VIII, each element in the column has the same number of valence electrons, giving them similar properties.
- Atoms that form cations (+ ions) tend to be smaller because there are less repulsion forces. Anions (- ions) tend to be larger because there are more repulsion forces.
