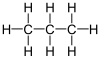
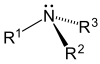
AP Chemistry/Organic Chemistry
Prefixes
[edit | edit source]| prefix | number of carbons | alkyl group |
| meth- | 1 | methyl |
| eth- | 2 | ethyl |
| prop- | 3 | propyl |
| but- | 4 | butyl |
| pent- | 5 | pentyl |
| hex- | 6 | hexyl |
| hept- | 7 | heptyl |
| oct- | 8 | octyl |
| non- | 9 | nonyl |
| dec- | 10 | decyl |
Examples
[edit | edit source]- is ethane
- is propane
- is octane
Alkyl groups
[edit | edit source]An alkyl group is a radical with a certain number of carbons and is of the general formula that has had one of its hydrogens removed, freeing up one of its bonds. This allows it to bond to a carbon (or oxygen, etc.) in a compound, and form more complex compounds.
Functional Groups
[edit | edit source]- Alcohols contain OH-, e.g. C2H5OH is ethanol or ethyl alcohol.
- Alkanes have the general formula of CnH2n+2 and are saturated (i.e. they only contain single bonds), such as methane(CH4 and propane (C3H8).
- Alkenes have the general formula of CnH2n and are unsaturated (i.e. they contain double/triple bonds), such as ethylene(C2H4).
- Alkynes have the general formula of CnH2n-2 and are unsaturated, such as ethylyne (C2H2).
- Amines contain nitrogen, and resemble ammonia structurally.
- Nitriles contain a nitrile group(CN-).
| Compound | Aldehyde | Ketone | Carboxylic acid | Ester | Amide |
| Structure |  |
 |

| ||
| General formula | RCHO | RCOR' | RCOOH | RCOOR' | RCONHR' |
Skeletal formulae
[edit | edit source]Skeletal formulae are very important in organic chemistry for two reasons: (1) they appear very frequently, and (2) they make sketching organic compounds much easier.
Consider the following image.
![]() The image to the left is butane (C4H10). As you can see however there are no Cs and Hs written on the sketch. Carbons are represented by the vertices at the ends of the line segments. The line segments themselves represent (as normal) single bonds. The hydrogens are not drawn in, but are there, because all carbons form four bonds. Because butane is saturated, the hydrogens have to attach to the carbons, where they can still form bonds.
The image to the left is butane (C4H10). As you can see however there are no Cs and Hs written on the sketch. Carbons are represented by the vertices at the ends of the line segments. The line segments themselves represent (as normal) single bonds. The hydrogens are not drawn in, but are there, because all carbons form four bonds. Because butane is saturated, the hydrogens have to attach to the carbons, where they can still form bonds.
![]() The image to the left is ethene (C2H4). As you can see, there are two line segments in this sketch. When two or three line segments appear superincumbently, they represent double and triple bonds respectively (as they do in the structural formulae).
The image to the left is ethene (C2H4). As you can see, there are two line segments in this sketch. When two or three line segments appear superincumbently, they represent double and triple bonds respectively (as they do in the structural formulae).
 This is another important concept in organic chemistry. Since its discovery thousands of new organic chemicals have been created, based on this one component. The formula for benzene is C6H6. Benzene occurs commonly in organic chemicals called aromatics, as seen to the right (in aspirin).
This is another important concept in organic chemistry. Since its discovery thousands of new organic chemicals have been created, based on this one component. The formula for benzene is C6H6. Benzene occurs commonly in organic chemicals called aromatics, as seen to the right (in aspirin).
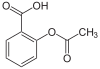 Carbon and hydrogen (although hydroxide (OH-) is written out as well) are the only two elements that are not indicated with their chemical symbol in skeletal formulae; they are called implicit. Other common elements in organic chemistry such as nitrogen, chlorine, fluorine, and oxygen are indicated with their chemical symbols; they are called explicit, and are seen to the right. Remember the symbol R is any alkyl group. Alkyl groups should be considered explicit.
Carbon and hydrogen (although hydroxide (OH-) is written out as well) are the only two elements that are not indicated with their chemical symbol in skeletal formulae; they are called implicit. Other common elements in organic chemistry such as nitrogen, chlorine, fluorine, and oxygen are indicated with their chemical symbols; they are called explicit, and are seen to the right. Remember the symbol R is any alkyl group. Alkyl groups should be considered explicit.
![]()
Other Naming Conventions
[edit | edit source]Naming alkanes, alkenes, and alkynes
[edit | edit source]- Determine if the compound is saturated or unsaturated
- If it is saturated, it is an alkane, and takes the suffix –ane
- If it is unsaturated
- And has double bonds, it is an alkene, and takes the suffix -ene
- And has triple bonds, it is an alkyne, and take the suffix –yne
- Find the main chain by counting the number of carbons starting at one end of the compound, and goint to another end, without counting a carbon twice (keep some of the later steps in mind). Then, based on this number of carbons select a prefix from the chart in the prefixes section above.
- If it is an alkane, just use the suffix from step 1.1.
- If it is an alkene, use the appropriate suffix from step 1.2. Then, place the number of the carbon where the double/triple bond begins, making it the lowest number possible, and separate it from the number-of-carbons prefix and suffix with hyphens (e.g. but-2-ene ). Note that C1 (the first carbon in the main chain) should be based on this step. It also seems to be acceptable to place the number before the name of the main chain (e.g. 2-butene)
- If the compound is an alkene, then another element can be added to the name.
- If the parts of the compound separated by the double bond are on the same side of the double bond, then the prefix cis- is added before the name of the main chain (e.g. cis-but-2-ene).
- If the parts of the compound separated by the double bond are on different sides of the double bond, then the prefix trans- is added before the name of the main chain (trans-but-2-ene).
- Identify if the compound has alkyl groups.
- If there are, determine how many there are, and where they are relative to C1.
- The number of the carbon where the alkyl group attaches to the main chain is placed before the name of the alkyl group, and this is placed before the name of the main chain (e.g. 2-methylbutane)
- If there are more than one type of alkyl group, the alkyl groups are named in alphabetical order, and separated by commas (e.g. 3-ethyl,4-methyhexane)
- If there are more than one of a certain type of alkyl group, the carbons where they attach are separated by commas, and a prefix based on their number is added (e.g. 2,2-dimethypropane). A list is provided below.
- If there are, determine how many there are, and where they are relative to C1.
Alkyl group prefixes
[edit | edit source]| Number of groups | prefix |
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
Examples
[edit | edit source]
- The compound is saturated, and therefore is an alkane.
- The main chain of carbons contains six carbons (hex-).
- It is not an alkene.
- The compound has one alkyl group (methyl) connecting at C2.
This compound’s name is 2-methylhexane

- The compound is saturated, and therefore is an alkane.
- The main chain contains four carbons (but-).
- It is not an alkene.
- The compound has two alkyl groups (both methyl) connected at the C2 and C3
The name of this compound is 2,3-dimethylbutane

- This compound is unsaturated and is an alkene (double bonds).
- The main chain has six carbons (hex-), and the double bond is at C3.
- Disregard step 3.
- The compound has no alkyl groups.
The name of this compound is hex-3-ene or 3-hexene.

- This compound is unsaturated and is an alkyne (triple bonds)
- The main chain has six carbons (hex-)
- Disregard step 3.
- the compound has no alkyl groups
The name of this compound is hex-1-yne or 1-hexyne.
Isomers
[edit | edit source]- Structural - carbons are arranged differently.
- Chain - carbon chain is different.
- Position - functional group is in a different position on the carbon chain.
- Functional group - functional group is different.
- Stereoisomers - isomers with the same structural formula but a different arrangement of atoms in space.