Structural Biochemistry/Proteins/Proteins
General Information
[edit | edit source]Proteins are important organic compounds that serve as structural elements, transportation channels, signal receptors and transmitters, and catalysts; they are the most versatile macromolecules found in living organisms. Protein compositions are made up of one or more polypeptides which are composed of combinations that are derived from the 20 different amino acid subunits. These polypeptides are linear polymer chains of amino acids that are bonded together by a peptide bond that is formed between carboxyl and amino groups of adjacent amino acid residues. Each amino acid has its own size, shape, and set of properties, and proteins have 50 to 2,000 amino acids connected end-to-end in many different combinations (Structures of Life 3). Proteins can have different functionalities and roles in the body due to many different possible structures and shapes. One specific characteristic of proteins is that only the L isomers of amino acids are found in nature and used in protein. There is no evidence that explains why this happens. Proteins have many different active functional groups attached to them to help define their properties and functions. Proteins perform a number of important functions, ranging from acting as very rigid structural elements to transmitting information between cells. In addition, complex assemblies are formed due to proteins reacting with each other and with other macromolecules. Proteins fold into secondary, tertiary, and quaternary structures based on the intramolecular bonding between functional groups and can take on a variety of three-dimensional shapes depending on the amino acid sequence.
One example of a protein is collagen, a fibrous structural protein that is the most abundant protein found in animals. The structure of collagen consists of a triple helix and consists of mainly three polypeptide chains held together by hydrogen bonds, similar to that of DNA's double helix. This structure of collagen was determined using the method of X-ray crystallography. There are several important properties that enable proteins to perform a variety of crucial functions.
1. LINEAR POLYMERS: Proteins are built out of monomer units (amino acids): Based on the sequence of amino acids, proteins spontaneously fold up into three-dimensional structures.
2. CONTAIN A WIDE RANGE OF FUNCTIONAL GROUPS: Proteins contain functional groups such as alcohols, thiols, thioethers, carboxylic acids, etc. These functional groups are key to the variety of functions the protein can perform.
3. PROTEIN INTERACTION FOR COMPLEX ASSEMBLIES: Within complex assemblies, proteins act synergistically in order to achieve a specific function.
4. STRUCTURE: Proteins vary in flexibility. Rigid units of a protein can function as structural elements in the cytoskeleton of cells or in connective tissue. Protein structure Is divided into four categories and is a crucial element in the specificity of protein function.
Proteins are usually portrayed in 3D structures. They are usually categorized into 4 different characteristics and levels:

Primary: The primary structure of a polypeptide is its amino acid sequence, from beginning to end. The primary structures of polypeptides are determined by encoding genes. Genes carry the information to make polypeptides with a defined amino acid sequence. An average polypeptide is about 300 amino acids in length, and some genes encode polypeptides that are a few thousand amino acids long.
Secondary: The amino acid sequence of a polypeptide, together with the laws of chemistry and physics, cause a polypeptide to fold into a more compact structure. Amino acids can rotate around bonds within a protein. This is the reason proteins are flexible and can fold into a number of shapes. Folding can be irregular or certain regions can give a repeating folding pattern. Such repeating patterns are called secondary structures. The two types are the α-helix and β-sheet. In an α-helix, the polypeptide backbone forms a repeating helical structure that is stabilized by hydrogen bonds. These hydrogen bonds occur at regular intervals and cause the polypeptide backbone to form a helix. In a β-sheet, regions of the polypeptide backbone come to lie parallel to each other. When these regions form hydrogen bonds, the polypeptide backbone forms a repeating zigzag shape called a β-sheet.
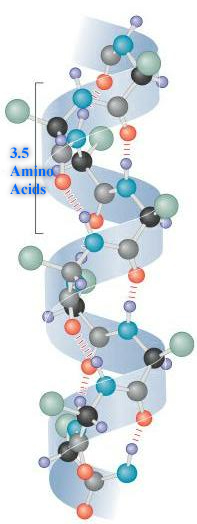
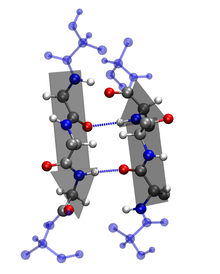
Tertiary: As the secondary structure becomes established due to the primary structure, a polypeptide folds and refolds upon itself to assume a complex three-dimensional shape called the protein tertiary structure. The tertiary structure is the three-dimensional shape of a single polypeptide. For some proteins, such as ribonuclease, the tertiary structure is the final structure of a functional protein. Other proteins are composed of two or more polypeptides and adopt a quaternary structure.
Quaternary:
Most functional proteins are composed of two or more polypeptides that each adopt a tertiary structure and then assemble with each other. The individual polypeptides are called protein subunits. Subunits can be identical polypeptides or can be different. When proteins consist of more than one polypeptide chain, they are said to have quaternary structure and are also known as multimeric proteins, meaning many parts.
These proteins bind in a specific shape through interactions such as hydrogen bonding, salt bridges, and disulfide bonds. The two major structure categories of proteins are fibrous and globular. An example of a fibrous protein is keratin, which is found in wool, hair, myosin and actin in muscles, fur, nails, and fibrinogen for blood clotting. Examples of a globular protein include insulin, hemoglobin, and most enzymes.

Factors that influence protein structure:
[edit | edit source]Several factors determine the way that polypeptides adopt their secondary, tertiary and quaternary structures. The amino acid sequences of polypeptides are the defining features that distinguish the structure of one protein from another. As polypeptides are synthesized in a cell, they fold into secondary and tertiary structures, which assemble into quaternary structures for most proteins. As mentioned, the laws of chemistry and physics, together with amino acid sequence, govern this process. Five factors are critical for protein folding and stability:
2. Ionic bonds and other polar interactions
Protein Recognition
[edit | edit source]Protein functions such as molecular recognition and catalysis depend on their complementary binding sites. They also depend on specialized microenvironments that result from protein's tertiary structure. Such specialized microenvironments at binding sites eventually contribute to catalysis. Binding sites have a diverse distribution of charges which allow the substrates to bind.
Protein Denaturing
[edit | edit source]
Upon addition of heat, proteins begin to denature. Denaturation occurs in the tertiary and secondary structures. If denaturation occurs, this could lead to protein inactivity, or even cause the cell to die and no longer function.
The reason that heat is able to cause the protein to denature is because it disrupts the bonds due to the rapid vibrations that it causes in these molecules.
Heat effects the tertiary and secondary structures. The primary structure of a protein is just peptide bonds, and heat is not strong enough to break these peptide bonds, so heat doesn't have an effect on the primary structure.
Protein Hormones
[edit | edit source]Leptin and Insulin
[edit | edit source]Hyperphagia as well as elevated levels of insulin and leptin are found in obesity although leptin is supposed to be a feeding inhibitor while lowering insulin levels and suppressing insulin production. Leptin may not be functioning as predicted due to the correlation found that Hyperphagia may cause leptin resistance. This could be related to insulin resistance as well. Leptin is a strong modulator of biochemical pathways and metabolic fluxes which in turn causes a redistribution of glucose fluxes. Research suggests that if leptin secretion at an early time due to overeating may have a correlation with obesity and glucose intolerance. Over feeding decreases the rate of glucose infusion needed to maintain regular glucose levels. Due to this, the intake of carbohydrates was drastically altered because after 7 days of over eating the rate of glucose intake was decreased. Over feeding drastically decreased insulin’s inhibition of glucose production. Voluntary over feeding decreases the extent to which leptin affects food consumption. In an experiment with over fed rats and rat control group, this was proved by injecting leptin to both groups. The group of over fed rats had no response to the leptin therefore their food intake did not decrease but the control group was seen to have the expected outcome of leptin. In the control group, the leptin functioned as expected and inhibited food intake. The increase in body mass due to increase in food intake may be related to causing insulin resistance as well as early increase in glucose production during hyperphagia. Therefore, it is proved that the increase in food consumption plays a role in the paralysis/ decrease of the leptin system and a decreased action of insulin on carbohydrate metabolism.
References
[edit | edit source]Matthew D. Shoulders and Ronald T. Raines. "Collagen Structure and Stability" http://www.annualreviews.org/doi/full/10.1146/annurev.biochem.77.032207.120833?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed "Quaternery Protein." Elmhurst College: Elmhurst, Illinois. Web. 12 Nov. 2011. <http://www.elmhurst.edu/~chm/vchembook/567quatprotein.html>. http://diabetes.diabetesjournals.org/content/50/12/2786.full.pdf+html
