Structural Biochemistry/Hemoglobin
General
[edit | edit source]
Hemoglobin (Haemoglobin in many varieties of English and often abbreviated to 'Hb') is a tetramer consisting of two dimers that bind to oxygen. Hemoglobin is the oxygen-transporting protein of red blood cells and is a globular protein with a quaternary structure. Hemoglobin consists of four polypeptide subunits; 2 alpha chains and two beta chains. Hemoglobin transports oxygen in the blood from the lungs to the rest of the body. The three-dimensional structure of hemoglobin was solved using X-ray crystallography in 1959 by Max Perutz. The structure of hemoglobin is very similar to the single polypeptide chain in myoglobin despite the fact that their amino acid sequences differ at 83% of the residues. This highlights a relatively common theme in protein structure: that very different primary sequences can specify very similar three-dimensional structures.
There are two states in the hemoglobin, the T state (the tense state) and the R state (the relaxed state). The T state has less of an affinity for oxygen than the R state. In the concerted mode of cooperativity, the hemoglobin must either be in its T state or R state. In the sequential mode of cooperativity, the conformation state of the monomer changes as it binds to oxygen. Actual experimental observation of hemoglobin shows that it is more complex than either of the models and is somewhere in between the two. The conformation of hemoglobin also changes as the oxygen binds to the iron, raising both the iron and the histidine residue bound to it. The oxygen binding changes the position of the iron ion by approximately 0.4 Å. Before oxygenation the iron ion lies slightly outside the plane of the porphyrin upon oxygenation it moves into the plane of the heme. The oxygen affinity of hemoglobin decreases as the pH decreases. This is useful because, with a high affinity for oxygen in the lungs, hemoglobin can effectively bind to more oxygen. Once it reaches the muscle, where the pH is lower, the lowered affinity for oxygen allows hemoglobin to release its oxygen into the tissues. When carbon dioxide diffuses into red blood cells, its dissociation also causes a decrease in pH.
Relative Affinity and Efficiency
[edit | edit source]The affinity of hemoglobin for oxygen is less than its structural analog myoglobin. Interestingly enough, however, this does not affect hemoglobin's usefulness for the body; on the contrary, it allows hemoglobin to be a more efficient oxygen carrier than myoglobin. This is so because hemoglobin can release oxygen more easily than can myoglobin. While it is important for oxygen to be carried to different areas of the body, it is even more important for the oxygen to be released when needed. The higher the affinity of a given protein for oxygen, the harder it will be for that protein to release oxygen when the time comes. Thus, hemoglobin's lower affinity for oxygen serves it well because it allows hemoglobin to release oxygen more easily in the body. Myoglobin, on the other hand, has a significantly higher affinity for oxygen and will, therefore, be much less inclined to release it once it is bound. Thus hemoglobin's lower affinity for oxygen relative to myoglobin allows it to have a higher overall efficiency in binding and then releasing oxygen species. For this reason, the body tends to use hemoglobin more often for oxygen-distributing purposes, although myoglobin is used as well, particularly for carrying oxygen to muscle cells. More can be read on myoglobin in the appropriate section.
Also worth mentioning is the fact that fetal hemoglobin has a noticeably higher affinity for oxygen than does maternal hemoglobin. This is of crucial importance during pregnancy in human females (and presumably in other pregnant mammalian females) because it allows the fetus to obtain much-needed oxygen during development. Basically, the hemoglobin present in the fetus is able to strip oxygen species from the maternal hemoglobin when the mother's blood comes into contact with fetal material. The portion of the mother's blood that does not touch the fetus transfers oxygen as normal to the mother's organ systems.
When oxygen is bound to hemoglobin, the color changes to crimson red. When oxygen is not bound, the color becomes a dark "rustic" shade of red [2] . Hemoglobin's affinity to oxygen increases as more oxygen is bound to it. The disassociation curve represents how hemoglobin is cooperative to oxygen with its sigmoidal shape. - The left shift shows an increase in oxygen affinity. Hemoglobin has a better chance to hold onto oxygen. This normally occurs with a change in environmental factors such as low temperature, low metabolism rate, and high pH.
- The right shift shows a decrease in affinity. Hemoglobin is more likely to release Oxygen. This is due to high temperature, high metabolism, and low pH.
While Hemoglobin has 4 subunits, Myoglobin has one subunit. It is the enzyme of oxygen storage within the cells (found in skeletal muscle cells). The reason muscles are red is because they contain large amount of myoglobin. Organisms such as diving mammals have very large amounts of myoglobin so that they can go for an extended period of time without breathing.
States
[edit | edit source]As mentioned above, hemoglobin exists in two distinct states: the T-state and the R-state. The T-state of hemoglobin is the more "Tense" of the two; this is the deoxy form of hemoglobin (meaning that it lacks an oxygen species) and is also known as "deoxyhemoglobin". The R-state of hemoglobin is more "Relaxed" and is the fully oxygenated form; it is also known as
"oxyhemoglobin."
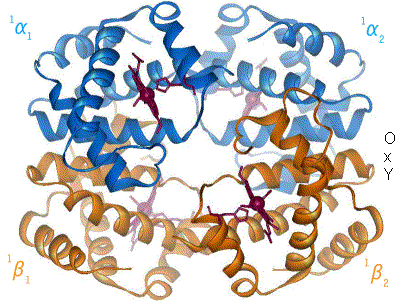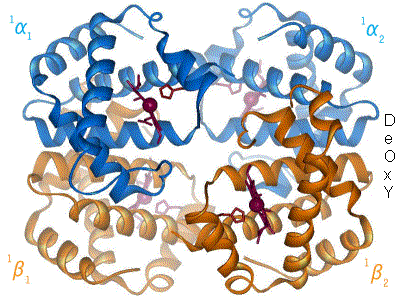
Cooperativity
[edit | edit source]One of the unique features of hemoglobin is that it exhibits cooperativity. This means that hemoglobin can transmit intramolecular messages to its various functional groups to help it attain a maximum affinity for the ligand of interest, which is oxygen in this case. When a monomer of hemoglobin binds to oxygen, it alerts other nearby hemoglobin monomers to start the binding process as well. This means that, as more and more oxygen is bound by hemoglobin monomers, the affinity of hemoglobin will increase more and more as well. In other words, the affinity of hemoglobin is proportional to the quantity of oxygen bound at a given time. This allows hemoglobin to increase its affinity for oxygen over time, a property that brands it as one of the most flexible proteins in the body. Because it can modify its affinity for oxygen, hemoglobin can exhibit a range of different affinities. As stated before, this makes it quite flexible in terms of how much oxygen it can bind and therefore how much it can release. This is one of the reasons that the body prefers to use hemoglobin, as opposed to myoglobin, for oxygen transport: hemoglobin can modify its own affinity for oxygen to suit the situation at hand, making it capable of handling a wider variety of chemical environments and organ systems while still being able to distribute oxygen effectively.
Models of Cooperativity
[edit | edit source]

There are two main models of cooperativity for hemoglobin. One of these is the concerted model of cooperativity. This model states that the hemoglobin molecule changes rapidly between its R- and T-states in order to maximize its affinity for oxygen. According to this model, hemoglobin is constantly "flipping" back and forth between states in an attempt to bind as much oxygen as possible. The other model is the sequential model of cooperativity. This model maintains that one strand of hemoglobin starts a sequence of conformational changes in hemoglobin that increase its affinity for oxygen. When one strand of hemoglobin binds oxygen, the hemoglobin rearranges in a manner that favors additional oxygen binding. When the next oxygen is bound, another conformational change occurs to further supplement binding; Thus, hemoglobin can sequentially increase its affinity for oxygen as more and more of its strands bind oxygen.
Experimental data obtained from kinetics experiments with hemoglobin reveals that neither the concerted nor the sequential model of cooperativity is heavily favored. If anything, the data suggests that hemoglobin's behavior represents a hybrid of the two models; thus hemoglobin's cooperativity is somewhere in between the concerted and sequential models.
It is known that hemoglobin undergoes several conformational changes upon binding with oxygen. First of all, as soon as the iron cation within hemoglobin begins to move, the Histidine residues and the alpha-helix of hemoglobin start moving as well to stabilize the changes caused by the movement of iron. Second, the carboxyl terminal end of the alpha-helix usually resides at the interface between the two alpha- and beta-dimers that make up hemoglobin. Finally, the positional changes of the carboxyl terminal end create favorable conditions for transitions between the T- and the R-states of hemoglobin.
The above description makes clear that the concerted and sequential models do not fully explain hemoglobin's behavior, nor the behavior of related classes of proteins. To account for this discrepancy, more complex models have been devised that more accurately reflect the kinetic data gained from experiments with hemoglobin binding.
Oxygen Binding Curve
[edit | edit source]Oxygen binding to iron in the heme group pulls part of the electron density from the ferrous ion to the oxygen molecule. It is important to leave the myoglobin in the dioxygen form rather than superoxide form when the oxygen is released because the superoxide can be generated by itself to have a new form that gives negative effect on many biological materials, and also the superoxide prevents the iron ion from binding to the oxygen in its ferric state (Metmyoglobin). Superoxide and superoxide-derived oxygen species are so reactive compared to the stable O2 molecule that they could have a destructive effect both within the cell and in its environment. A distal histidine residue in myoglobin regulates the reactivity of the heme group to make it more suitable for oxygen binding. It does this by H-bonding with the oxygen molecule; the additional electron density of the oxygen molecule makes the H-bond unusually strong and therefore even more effective as a stabilizing agent.
An oxygen-binding curve is a plot that shows fractional saturation versus the concentration of oxygen. By definition, fractional saturation indicates the presence of binding sites that have oxygen. Fractional saturation can range from zero (all sites are empty) to one (all sites are filled). The concentration of oxygen is determined by partial pressure.
Hemoglobin's oxygen affinity is relatively weak compared to myoglobin's affinity for oxygen. Hemoglobin's oxygen-binding curve forms in the shape of a sigmoidal curve. This is due to the cooperativity of the hemoglobin. As hemoglobin travels from the lungs to the tissues, the pH value of its surroundings decrease, and the amount of CO2 that it reacts with increases. Both these changes causes the hemoglobin to lose its affinity for oxygen, therefore making it drop the oxygen into the tissues. This causes the sigmoidal curve for hemoglobin in the oxygen-binding curve and proves its cooperativity.
 File:Oxygen binding curve with hemoglobin and myoglobin.jpg
File:Oxygen binding curve with hemoglobin and myoglobin.jpg
This image shows hemoglobin's oxygen binding affinity compared with myoglobin's affinity and the hypothetical curve that hemoglobin would have to follow if it did not show cooperativity. In this graph, you can see hemoglobin's sigmoidal curve, how it starts out with a little less affinity than myoglobin, but comparable affinity to oxygen in the lungs. As the pressure drops and the myoglobin and hemoglobin move towards the tissues, myoglobin still attains its high affinity for oxygen, while hemoglobin, because of its cooperativity, suddenly loses its affinity, therefore making it the better transporter of oxygen than myoglobin. The gray curve, showing no cooperativity, shows that to have the low affinity for oxygen needed in the tissues, the hemoglobin would have started with a smaller affinity for oxygen, therefore making it less efficient in bringing oxygen in from the lungs.
Oxygen Binding Curve for Hemoglobin
[edit | edit source]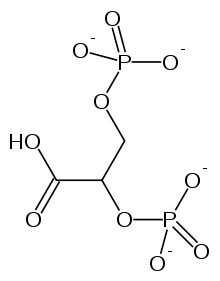
In red blood cells, the oxygen-binding curve for hemoglobin displays an “S” shaped called a sigmoidal curve. A sigmoidal curve shows that oxygen binding is cooperative; that is, when one site binds oxygen, the probability that the remaining unoccupied sites that will bind to oxygen will increase.
The importance of cooperative behavior is that it allows hemoglobin to be more efficient in transporting oxygen. For example, in the lungs, the hemoglobin is at a saturation level of 98%. However, when hemoglobin is present in the tissues and releases oxygen, the saturation level drops to 32%; thus, 66% of the potential oxygen-binding sites are involved in the transportation of oxygen.
Purified hemoglobin binds much more tightly to the oxygen, making it less useful in oxygen transport. The difference in characteristics is due to the presence of 2,3-Bisphosphoglycerate(2,3-BPG) in human blood, which acts as an allosteric effector. An allosteric effector binds in one site and affects binding in another. 2,3-BPG binds to a pocket in the T-state of hemoglobin and is released as it forms the R-state. The presence of 2,3-BPG means that more oxygen must be bound to the hemoglobin before the transition to the R-form is possible.
Other regulation such as the Bohr effect in hemoglobin can also be depicted via an oxygen-binding curve. By analyzing the oxygen-binding curve, one can observe that there is a proportional relationship between affinity of oxygen and pH level. As the pH level decreases, the affinity of oxygen in hemoglobin also decreases. Thus, as hemoglobin approaches a region of low pH, more oxygen is released. The chemical basis for this Bohr effect is due to the formation of two salt bridges of the quaternary structure. One of the salt bridges is formed by the interaction between Beta Histidine 146 (the carboxylate terminal group) and Alpha Lysine 40. This connection will help to orient the histidine residue to also interact in another salt bridge formation with the negatively charged aspartate 94. The second bridge is form with the aid of an additional proton on the histidine residue.
As carbon dioxide diffuses into red blood cells, it reacts with water inside to form carbonic acid. Carbonic acid dissociated leads to lower pH and stabilizes the T state.
An oxygen-binding curve can also show the effect of carbon dioxide presence in hemoglobin. The regulation effect by carbon dioxide is similar to Bohr effect. A comparison of the effect of the absence and presence of carbon dioxide in hemoglobin revealed that hemoglobin is more efficient at transporting oxygen from tissues to lungs when carbon dioxide is present. The reason for this efficiency is that carbon dioxide also decreases the affinity of hemoglobin for oxygen. The addition of carbon dioxide forces the pH to drop, which then causes the affinity of hemoglobin to oxygen to decrease. This is extremely evident in the tissues, where the carbon dioxide stored in the tissues are released into the blood stream, then undergoes a reaction that releases H+ into the blood stream, increasing acidity and dropping the pH level.
File:Reason carbon dioxide decreases pH.jpg
Allosteric effectors of hemoglobin
[edit | edit source]Allosteric regulation is the process by which the behavior of proteins is controlled by other molecules; the molecules that perform this regulation are known as allosteric regulators. This process involves the binding of an allosteric regulator molecule to the protein in question; the result is a distinct effect on the protein's function. Allosteric regulators that increase or supplement a given protein's function are known as allosteric activators. Those that decrease or interrupt a given protein's function are known as allosteric inhibitors.
Hemoglobin, like other proteins, has its share of allosteric regulators. Regulation is highly necessary for a protein as important as hemoglobin, since its affinity for oxygen must be just right for the particular organ system that it is currently dealing with. Thus the main concern for most of hemoglobin's allosteric regulators is tweaking its oxygen affinity to match the situation at hand.
The advantages of cell using allosteric inhibitors are: - In a typical metabolic pathways, the final product of the pathway acts as an allosteric inhibitor. - It inhibits the 1st enzyme in the pathway saving the cell from using resources in a metabolic pathway which final product is abundant.
Bisphosphoglycerate, or BPG, is one of many allosteric regulators for hemoglobin. This molecule binds to the central cavity of the deoxyhemoglobin version of hemoglobin (T-state) and stabilizes it. The increased stability of the T-state results in a decreased affinity for oxygen, since normally it is the intense straining of the T-state that drives deoxyhemoglobin to bind to oxygen; once oxygen is bound, the T-state loses its strain and relaxes into the R-state. Thus, by stabilizing the normally tense T-state, BPG makes hemoglobin less likely to bind oxygen in an attempt to release the strain. This mechanism is necessary, because the T state of hemoglobin is so unstable that the equilibrium lies very strongly in favor of the R state and little to no oxygen is released. In other words, pure hemoglobin binds to oxygen very tightly. 2,3-BPG was thus needed to stabilize the T state. Because BPG decreases hemoglobin's affinity for oxygen, it is an allosteric inhibitor of hemoglobin. Without 2, 3-BPG, hemoglobin would be an extremely inefficient transporter of oxygen from the lungs to the tissues, releasing only about 8% of its oxygen content. However, in the presence of 2,3-BPG, more oxygen-binding sites in the hemoglobin tetramer must be filled in order to transition from the T to the R state. Higher concentrations of oxygen must be reached in order for hemoglobin to transition from the lower-affinity T-state to the higher-affinity R state.
The binding of 2,3-BPG has further physiological consequences. Fetal hemoglobin has a higher oxygen-binding affinity than that of maternal hemoglobin. Fetal red blood cells have a higher affinity for oxygen than maternal red blood cells because fetal hemoglobin doesn't bind 2,3-BPG as well as maternal hemoglobin does. The result of this difference in oxygen affinity allows oxygen to be transferred effectively from maternal to fetal red blood cells.
The pH, or proton concentration of a given solution, is another allosteric regulator of hemoglobin. Interestingly enough, pH can act as both an allosteric activator and inhibitor, depending on the direction of pH change. As pH decreases, for example, the affinity of hemoglobin for oxygen decreases as well. This is due to the fact that protons help construct salt bridges in the T-state of hemoglobin. In general, the T-state of hemoglobin is favored by three amino acids that form two salt bridges; one of these salt bridges requires an added proton to form successfully. Thus, the higher the proton concentration (or the lower the pH) in the solution, the easier this salt bridge will form. Better salt bridge formation leads to a better and more stable T-state, and as mentioned before, a more stable T-state means decreased oxygen affinity of hemoglobin. Since higher proton concentration corresponds to lower pH, this means that the lower the pH, the more stable the T-state will be. Finally, the more stable the T-state, the lower the affinity for oxygen will be in hemoglobin molecule; thus pH acts as an allosteric inhibitor of hemoglobin when it is decreasing. Logically, then, the opposite effect would occur when the pH increases. This would signify a lower proton concentration, meaning more difficult salt bridge formation and thus a slower-forming and less stable T-state. A less stable T-state would be much more inclined to bind with oxygen; thus increased pH results in increased oxygen affinity for hemoglobin. The result is that pH acts as an allosteric activator for hemoglobin when it is increasing.

Carbon dioxide, or CO2, is yet another allosteric inhibitor of hemoglobin. There are several reasons for this. The first is that the enzyme carbonic anhydrase can help carbon dioxide react with water to form carbonic acid, which dissociates into bicarbonate and a proton. With enough carbonic anhydrase enzymes present, therefore, carbon dioxide can cause a decrease in the pH of the solution due to all the protons produced from its reaction with water. As mentioned in the previous paragraph, more protons means decreased pH, which in turn means a decreased affinity of hemoglobin for oxygen. Carbon dioxide also neutralizes the positive charge on the amino terminus of hemoglobin (amino groups usually exist in their protonated forms in living systems). This charge neutralization results in production of negatively charged carbamate groups, which form salt bridges that lead to stabilization of the T-state of hemoglobin, which results in a decreased affinity for oxygen. Thus carbon dioxide functions as an effective allosteric inhibitor of hemoglobin.
Bohr effect
[edit | edit source]Bohr effect is a property of hemoglobin which states that at lower pH (more acidic environment), hemoglobin will bind to oxygen with less affinity. Since carbon dioxide is in direct equilibrium with the concentration of protons in the blood, increasing blood carbon dioxide levels leads to a decrease in pH, which ultimately leads to a decrease in affinity for oxygen by hemoglobin.
Physiological role The Bohr effect facilitates oxygen transport. Hemoglobin binds to oxygen in the lungs and releases it in the tissues predominately to those tissues in most need of oxygen. When a tissue's metabolic rate increases, its carbon dioxide production increases. Carbon dioxide forms bicarbonate through the follow reaction:
- CO2 + H2O H2CO3 H+ + HCO3−
This reaction usually progresses very slowly. With the help of the enzyme carbonic anhydrase, the formation of bicarbonate and protons in the red blood cells is accelerated. This causes the pH of the tissue to decrease and promote the dissociation of oxygen from hemoglobin to the tissue, allowing the tissue to obtain enough oxygen to meet its demands. Conversely, in the lungs, oxygen concentration is high. The binding of oxygen causes hemoglobin to release protons, which combine with bicarbonate to drive off carbon dioxide in exhalation. Since these two reactions are closely matched, there is little change in blood pH.


BPG binds to hemoglobin and affect oxygen binding: BPG binds in the central cavity of T-state hemoglobin. The anion groups of BPG are within Hb-bonding and ion-paring distances of the N-terminal amino group of both b subunits. BPG binds to and stabilizes only the T-state hemoglobin. This shifts the T R equilibrium toward the T state, which lowers the O2 binding affinity. BPG is really important for O2 transport in our body. One example is high altitude adaptation. High altitude will induce a rapid increase in the amount of BPG synthesized in erythrocytes. The increased amount of BPG will shift the oxygen binding curve from sea-level position to a lower affinity position (shift to right). This decreases the amount of O2 binding in the lungs, but, to a greater extent, increases the amount of O2 released at tissues. So hemoglobin can deliver more O2 from lungs to tissues.
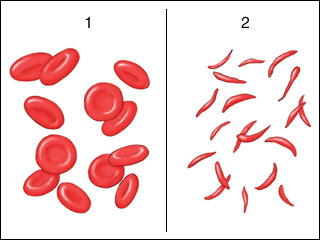
Sickle cells can cause hemoglobin cells which transport oxygen to the heart and parts of body change their shapes. It makes the transportation happens not smoothly and cause a disease.
Sickle Cell Anemia
[edit | edit source]A disease that affects many individuals's hemoglobin functionalities is sickle cell anemia, which cause by substitution of Valine instead of Glutamate at position 6 in amino acid sequence. Symptoms occur when an individual is several months old. Sickle cell anemia is characterized by decreased breath intake, delayed growth and development, fever, jaundice, rapid heart rate, and many other ailments. The problem is that hemoglobin in these indivudals are mutated. This mutated form of hemoglobin is called hemoglobin S and is less soluble than regular hemoglobin forms. Examination of the structure of hemoglobin S reveals that a new valine residue lies on the surface of the T-state molecule. As a result of this change deoxyhemoglobin has a hydrophobic patch on its surface. The hydrophobic patch interacts with other hydrophobic patches causing the molecule to aggregate into strands that align into insoluble fibers. Because this mutated form cannot move freely when they accumulate in the blood stream they end up rupturing or distorting the shape of the red blood cells when delivering oxygen. The red blood cells end up becoming a sickle or crescent shape. These inflicted cells are much less efficient in deliver oxygen through the body's circulation. They can clog fairly easily in smaller areas of blood flow causing them to disrupt blood flow. Sickle cell anemia should not be mistaken with hemophilia which is a disease in which an individual's body cannot form blood clots. If gone without proper treatment, people with this disease usually die from organ failure from ages 20 to 40. Better technology and data on this disease has led to treatments that involve folic acid supplements that activate the production of new healthy red blood cells. Treatment must be ongoing and is meant to limit the number of pains and emergencies of the disease. Overall immune system also suffers from this disease so often people take antibiotics and vaccines to prevent themselves from getting sick.
Sickle cell anemia is passed down through families and a child can only receive sickle cell anemia if both parents also have the disease. About 1 in 12 African Americans have this trait. There is a significant correlation between regions with high frequency of sickle cell anemia and regions with high prevalence of malaria. People with the sickle cell trait are resistant to malaria because the parasite that carries the disease needs to live within a red blood cell at some point in its life and is unable to survive in a sickle cell. Therefore, due to natural selection overtime the number of people with sickle cell anemia grew because before there was a cure for malaria the majority of the people who got malaria would die. It is now possible to diagnose sickle cell anemia during pregnancy. Patients with the disease are encouraged to drink enough fluids, get enough oxygen, responding quickly to infections. Strenuous physical activity should be avoided, smoking should be avoided, and too much sun exposure should also be avoided. Extreme consequences of sickle cell anemia no doubt includes death but others include blindness, spleen malfunction, tissue death, strokes, and acute chest pains. Below are pictures comparing healthy red blood cells to blood cells inflicted by sickle cell anemia.
Thalassemia
[edit | edit source]Just as sickle cell anemia is a difference in one amino acid, thalassemia is also an inherited disease where there is a reduction or loss of a hemoglobin chain. This leads to lower levels of hemoglobin and those with the disease experience anemia, fatigue, pale skin as well as spleen and liver malfunctions. Thalassemia branches into two different types: α-thalessemia and β-thalessemia. In α-thalessemia, the α-helix of hemoglobin is in low supply. This makes hemoglobin with high affinity for oxygen and no cooperativity therefore, making the release of oxygen in tissue poor. This is caused by a disruption in 4 alleles on chromosome 16 and is more rare. In β-thalessemia, the β-chain is in low supply. the extra α-helixes will aggregate and precipitate inside red blood cells which can result in anemia. β-thalessemia is caused by disruption on two alleles on chromosome 11.
Carbon Monoxide Poisoning
[edit | edit source]Carbon Monoxide (CO) is a dangerous gas because it is odorless and colorless. Sources of carbon monoxide include running automobiles and gas-powered appliances. When inhaled, it binds at the same sites as oxygen and can negatively impact the body's ability to absorb oxygen. Carbon monoxide binds to hemoglobin 200 times more tightly than oxygen does. Even at low partial pressures, carbon monoxide will prevent hemoglobin from delivering oxygen to the body. Once carbon monoxide binds to one site of hemoglobin, hemoglobin turns into the R-state which increases oxygen affinity and prevents oxygen dissociation in tissues.
Treatment of carbon monoxide poisoning includes the administration of 100% oxygen at higher partial pressures. Because of the higher pressure, this will displace most of the carbon monoxide from hemoglobin.
Breathing of 100% O2 helps reduce the half-life of COHb, Carboxyhemoglobin, a stable complex of CO and hemoglobin formed in red blood cells with the presence of CO. Measurement of COHb level in red blood cells is used to confirm exposure to CO and assess the severity of poisoning. Elevated level of COHb is determined more than 2% for nonsmokers and more than 9% for smokers.
By replacing oxygen in hemoglobin, CO cuts off the supply of oxygen to tissues and cells, which can result in neurological problems in adults, learning disabilities and developmental issues in children, and miscarriage in women during pregnancy.
CO poisoning symptoms are not obvious, including headache, dizziness, nausea, fatigue and weakness. They can be mistaken as food poisoning, influenza, migraine headache, or substance abuse.
2 main types of CO poisoning: acute, caused by exposure to high level of CO in a short period of time, and chronic or subacute, caused by exposure to low level of CO in a long period of time.
Impact of CO poisoning on body systems
- Neurologic: central nervous system depression, causing headache, dizziness in mild cases and coma, seizure in severe cases.
- Cardiac: decreased myocardial functions, vasodilatation, and decreased oxygen delivery and utilization by myocardium, causing chest pains, low blood pressure, fast heart rates.
- Metabolic: hyperventilation in mild cases, metabolic acidosis in severe cases.
- Pulmonary: pulmonary edema occurs in 10-30% of acute cases.
- Multiple organ failure: happens at high level of CO poisoning.
Fetal hemoglobin is the main oxygen transport protein. It happens during the last 7 months of development until 8 months later
Fetal Hemoglobin
[edit | edit source]
A fetus obtains its source of oxygen from the mother’s lungs. The oxygen in the mother’s bloodstreams attaches to hemoglobin molecules in the red blood cells and diffuses to the fetal bloodstream at the placenta. By the time the blood reaches the fetus, the pressure is much lower, which is not enough for a normal adult.
During the entire fetal formation period, three different types of hemoglobin are produced, with the succeeding hemoglobin deactivating its predecessor. All three types have the same heme molecules and iron atom, but differ slightly in structure. In the first eight weeks, majority of the hemoglobin is a type called embryonic hemoglobin. The production of Hemoglobin follows by the fetal hemoglobin (Hemoglobin F). It is the predominant form of hemoglobin expressed in the fetus development. The Hemoglobin F is apparent weeks after conception until a few months after birth. Around the thirty-fifth week, the adult hemoglobin (Hemoglobin A) starts production. Eventually, the blood cells will only contain Hemoglobin A, which is the only one produced for the duration of the human life.
Structural differences between the adult hemoglobin and the fetal hemoglobin
From the structural point of view, the adult hemoglobin is composed of 4 heme groups, 2 alpha chains and 2 beta chains. The fetal hemoglobin (also termed haemoglobin F) is also composed of 4 heme groups, 2 alpha chains and 2 gamma chains. The gamma chains are referred to as gamma subunits, which are homologous to the beta chains of the adult hemoglobin. In addition, the fetal hemoglobin and adult hemoglobin are found to be different near the 2,3 BPG binding site. The 2,3 BPG binds less tightly with the deoxy form of fetal hemoglobin as compared to the deoxy form of adult hemoglobin.
Additionally, another form of haemoglobin, termed haemoglobin A2, and comprising of two alpha and two delta globin chains; is produced in small quantities throughout childhood and adulthood. Haemoglobin A2 accounts for around 2-3% of total haemoglobin levels.
Blood Doping
[edit | edit source]There is also a hormone that can induce the increase of red blood cell production. Erythopoietin is a glycoprotein hormone that controls erythropoiesis, or otherwise known as red blood cell production. It is a protein signaling molecule (cytokine) for erythocyte (red blood cell) precursors in the bone marrow. This hormone is produced in the interstitial fibroblasts in the kidney and in the perisinusoidal cells in the liver.
References
[edit | edit source]Biology, Eighth Edition. Pearson, Benjamin Cummings, 2008.
Berg, Jeremy. Biochemistry. 7. W.H. Freeman Company, 2011.
David Hames, Nigel Hooper. Biochemistry. 3rd edition. New York.Taylor and Francis Group, 2005.
http://www.mdconsult.com/das/article/body/305467842-2/jorg=journal&source=&sp=20829759&sid=0/N/818895/s0735675707006584.pdf?issn=0735-6757 http://www.testbreath.com/carbon_monoxide_in_breath.asp http://www.atsdr.cdc.gov/toxprofiles/tp201-c7.pdf http://obitet.gazi.edu.tr/makale/makale/internalcombustionengines/060.pdf http://www.physio-control.com/uploadedFiles/learning/clinical-topics/Detecting%20Carbon%20Monoxide%20Poisoning%20in%20the%20Emergency%20Dept.pdf http://sickle.bwh.harvard.edu/hbsynthesis.html