Structural Biochemistry/Catalysis
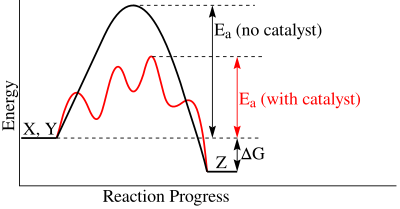
One of the most common functions of enzyme is the ability to catalyze reactions. During a reaction, the reactants must overcome activation energy in order for it to produce the products. The amount of activation energy needed determines how long the reaction takes to proceed. The lower the activation energy makes the faster rate of the reaction. The role of enzymes in catalyzing reactions is to stabilize the intermediate species, which is at the highest point of the activation energy, and thus dropping the activation energy. The enzyme is complementary not to the substrate but its intermediate state. If the enzyme binds to the substrate, it actually increases the activation energy. The equilibrium achieved is the same with or without the catalytic enzyme. However, what is affected is the time and rate in which it is achieved.
Generally, the higher the concentration of the substrate, the easier it is for the enzyme to bind to it. By plotting the amount of product produced as a function of time, the slope is how fast the reaction happens before the amount of substrate is saturated. This value is called the V0. Increasing the substrate concentration will increase the V0. However, there is a certain point in which the substrate concentration is too high and the reaction will not proceed any faster. This point is called the maximum velocity, Vmax. Every enzyme has their unique Vmax value. Another important identity of an enzyme is the Km value, defined as the substrate concentration at half of Vmax. Km is also unique to each enzyme. The turnover rate, the rate at which products are produced is called the Kcat. Dividing Kcat by Km gives the efficiency constant of the enzyme, which tells how fast the reaction is carried out and how likely the enzyme is to find the substrate. For more information, refer to Catalysis.
Phosphoryl-Transfer Reaction: Mechanisms and Catalysis
[edit | edit source]A key feature of phosphoryl-transfer reactions is that they often have extremely slow nonenzymatic rates and thus require large reaction rate accelerations using catalysts. The reactions that occur at the phosphorous atom of the phosphate ester also form the chemical basis for many of the most important and fundamental processes in living systems because they allow for the inheritance of genetic information through nucleic acids and are also responsible for using energy coupling to drive thermodynamically unfavorable reactions crucial to maintaining cell health and vitality. Phosphoryl transfer reactions also play an important role in metabolic pathways and signal transduction as well.
One possible mechanism for catalysis in these phosphoryl transfer reactions is the hydrolysis of phosphate monoesters. The hydrolysis rate often increases significantly as the pH decreases; this change indicates that the protonated form of the phosphate monoesters react much more quickly than the phosphate monoester dianions. The fact that these reactions are often carried out rapidly, with the help of enzymes, can be attributed to several factors. For example, the activation of the nucleophile can be accomplished in one of three ways; the way that the nucleophile is positioned can affect the nucleophile by increasing or even decreasing it. Another way is to reduce the electrostatic repulsion. One of the most important features of enzymes is their ability to use the binding interactions and positioned groups to carry out catalysis. The ability of enzymes to be able to accomplish this directly combines enzyme specificity with catalysis.
In phosphoryl transfer reactions, it’s also important that the nucleophile is aligned with the phosphorous atom as well as the leaving group for attack at phosphorous. Another factor that can contribute to the catalysis of monoester reactions is the stabilization of the negative charge on the potential leaving group. It has also been found that the transition states for phosphoryl transfer reactions can often be loose, tight, or synchronous depending on whether the compounds are phosphate monoesters, diesters, or triesters. Phosphate monoester usually proceeds through loose transition states, diesters through synchronous transition states, and triesters through tight transition states.
The presence of positively charged functional groups in the enzymes used in phosphoryl transfer reactions can also affect the reaction’s interactions with the oxygen atoms of the transferred phosphoryl group.
References
[edit | edit source]- Berg, Jeremy; Tymoczko, John; Stryer, Lubert. Biochemistry, 6th edition. W.H. Freeman and Company. 2007.
Jonathan K. Lassila,Jesse G. Zalatan, and Daniel Herschlag. "Biological Phosphoryl-Transfer Reactions: Understanding Mechanism and Catalysis". http://www.annualreviews.org/doi/full/10.1146/annurev-biochem-060409-092741?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme
http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Activation_energy
http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme_Catalytic_Mechanism/Catalysis