Statistical Thermodynamics and Rate Theories/Printable version
| This is the print version of Statistical Thermodynamics and Rate Theories You won't see this message or any elements not part of the book's content when you print or preview this page. |
The current, editable version of this book is available in Wikibooks, the open-content textbooks collection, at
https://en.wikibooks.org/wiki/Statistical_Thermodynamics_and_Rate_Theories
Molecular Energy Levels
To predict the properties of gases from their molecular properties, we must first know the different states the molecule can occupy and their energy levels.
Degrees of freedom
Molecular degrees of freedom refer to the number of ways a molecule in the gas phase may move, rotate, or vibrate in space. Three types of degrees of freedom exist, those being translational, rotational, and vibrational. The number of degrees of freedom of each type possessed by a molecule depends on both the number of atoms in the molecule and the geometry of the molecule, with geometry referring to the way in which the atoms are arranged in space. The number of degrees of freedom a molecule possesses plays a role in estimating the values of various thermodynamic variables using the equipartition theorem. These molecular degrees of freedom essentially describe how a molecule is able to contain and distribute its energy.
| Degree of freedom | Monatomic | Linear molecules | Non-linear molecules |
|---|---|---|---|
| Translational | 3 | 3 | 3 |
| Rotational | 0 | 2 | 3 |
| Vibrational | 0 | 3N - 5 | 3N - 6 |
| Total | 3 | 3N | 3N |
Translational degrees of freedom
[edit | edit source]Translational degrees of freedom arise from a gas molecule's ability to move freely in space. A molecule may move in the x, y, and z directions of a Cartesian coordinate system, appearing at a new position in space (relative to a starting position) via translation. A gas molecule is not restricted in which direction it may move, thus it has three translational degrees of freedom. This holds true for all gas molecules, whether they are monatomic, diatomic, or polyatomic, as any molecule may move freely in all directions in three-dimensional space.
Rotational degrees of freedom
[edit | edit source]A molecule's rotational degrees of freedom represent the number of unique ways the molecule may rotate in space about its center of mass which a change in the molecule's orientation. These axes may pass through atoms or bonds. By examining the molecule's symmetry (and, by extension, its geometry), the number of rotational degrees of freedom can quickly and easily be determined. A monatomic gaseous molecule such as a noble gas possesses no rotational degrees of freedom, as the center of mass sits directly on the atom and no rotation which creates change is possible.
A diatomic molecule, like H2 or HCl, has two rotational degrees of freedom. The center of mass of a linear molecule rests somewhere between the two terminal atoms. In the case of HCl it exists somewhere along the bond. The center of mass can be taken as the origin of a three-dimensional Cartesian grid, the z axis of which runs along the bond and through the two atoms. Rotation about the x and y axes generates a noticeable change in the molecule's orientation, while rotation about the z axis (analogous to the monatomic case) produces no change in the molecule and is considered a 'lost' rotational degree of freedom.
A polyatomic molecule may have either two or three rotational degrees of freedom, depending on the geometry of the molecule. For a linear polyatomic, such as CO2 or C2H2, the molecule has only two rotational degrees of freedom. The reason for this is discussed in the previous paragraph.
Vibrational degrees of freedom
[edit | edit source]The number of vibrational degrees of freedom (or vibrational modes) of a molecule is determined by examining the number of unique ways the atoms within the molecule may move relative to one another, such as in bond stretches or bends. This can be determined mathematically using the rule, where is the number of atoms in the molecule and is the number of vibrational degrees of freedom. Note that for linear molecules, the rule instead becomes , meaning a linear polyatomic made up of atoms will have one more vibrational degree of freedom than a non-linear polyatomic with atoms. Application of the rule for linear molecules shows that a diatomic molecule has only one vibrational degree of freedom. This is a logical conclusion, as there is only one bond vibration possible, a stretch of the bond between the two atoms.
- The three normal vibrational modes of water: bending (left), symmetric stretch (centre), and asymmetric stretch (right).
As an example, water, a non-linear triatomic, should have three vibrational degrees of freedom, since . The three vibrational modes corresponding to these three vibrational degrees of freedom can be seen above. These vibrational modes were determined computationally.
Translational energy
A gas particle can move through space in three independent directions: x, y, and z. Each of these directions is a distinct translational degree of freedom. For ideal gases, these particles can move freely through space in any of these directions until it collides with the wall of its container. For isolated systems, collisions with the wall of the container are assumed to be elastic, meaning no energy is lost upon impact.
Particle in a 1D Box
[edit | edit source]The first quantum mechanical model used for describing translation is a particle in a simple 1D box. It is free to move anywhere along one axis (usually assigned to be the x axis) between the arbitrarily assigned boundary limits 0 and a. At distances smaller than 0 and greater than “a” the potential energy function immediately rises to infinity. The particle cannot move past these points. 0 and a represent the walls of the 1D box. We can mathematically assign boundary conditions using this information which allows the derivation of the wave function for a particle in a 1D box. The resulting piece-wise function, wave function and energy level equation are given as follows,
As well as the corresponding energy levels of the system.
Note: In the equations above m represents the mass of the gas particle, and h is Planck's constant.
Zero-Point Energy
[edit | edit source]The energy levels for translation within the box are quantized, only discrete energy levels can be occupied by the particle at any given time. Each of these energy levels are defined by a single quantum number n. n can take on any integer values starting from 1 and up to a hypothetical infinity. The n = 0 state for a particle in a box does not exist. The particle, in accordance with the Heisenberg uncertainty principle cannot be motionless. If this were to be the case then both the momentum and position of the particle could be determined simultaneously, which is a violation of the principle. The energy of a particle in a 1D box at the lowest translational energy level is therefore non-zero (i.e., n = 1).
Probability Density Plots and the Correspondence Principle
[edit | edit source]It is possible to construct a probability density plot of the particle in a 1D box wave function. The plot is characterized by sizable “humps” at low values of n. For example, at n=1 there is one large hump which spans from a minimum at x = 0, a maximum somewhere in the center and a second minimum at x = a. These minimums represent regions of zero probability density. In other words, the particle will not be found in these regions.
As n increases the spacing between humps of the probability density plot becomes smaller and smaller until a near continuum is achieved at sufficiently high values of n. It must also be noted that the magnitude of the energy spacing’s between translational energy levels is extremely small relative to the amount of energy available to a particle under normal conditions. At room temperature ideal gas particles will occupy very high energy levels. As we increase the quantum number to a large enough value the behavior of the system will begin to reproduce that of classical mechanics in that the particle is essentially equally likely to be found anywhere in the box rather than within discrete hump regions seen at lower values of n. This is what is known as the correspondence principle.
Particle in a 3D Box
[edit | edit source]The correspondence principle applies to a 3D box as well. For a particle in a 3D box, the particle is able to move in any direction along the x, y and z axes. Since its motion now incorporates a combination of three possible directions the system must include two additional quantum numbers to compensate for the difference. nx, ny, and nz for the x, y, and z dimensions, respectively. Both the wave function and the equation for the energy levels must be adjusted to compensate for the new quantum numbers. These are given as follows,
a, b, and c represent the length of each corresponding side of the box. If the box were a cube then the energy level equation can be simplified because sides a, b, and c of the cube are all equal. The resulting equation would take on the form,
For a particle in a cube some combinations of quantum numbers will give the same energy level. Energy levels with a different set of quantum numbers but having the same energy are said to be degenerate and unless all three of the quantum numbers are identical there is always another combination of the three quantum numbers that will give rise to a degenerate state.
Energy Level Spacings, Degeneracy, and Density of States
[edit | edit source]Example
[edit | edit source]Calculate the energy difference, , for the translation of N2 from the ground state to the first excited state. Assume the box is a cube with an edge length of 10 cm.
Solution
[edit | edit source]The ground state, where , has a degeneracy of 1. The first excited state has a degeneracy of 3, involving the different combinations of quantum numbers and 2. Can be determined by using the following equation:
However because all edges of the cube is 10 cm, then a=b=c=10 cm = 0.1 m. Then the equation becomes:
The mass of N2 can be determined with the following equation:
Using the mass the can now be determined.
Further Reading
[edit | edit source]Wikipedia entry on particle in a box models
Rotational energy
Rotational Energy
[edit | edit source]Molecules that consist of more than one atom have rotational angular momentum. The rotational motion which is generated by these molecules is purely kinetic as each molecule rotates around the molecules centre of mass. A good approximation which makes interpreting the rotational energy levels easier is the rigid rotor approximation which is mentioned below. Essentially this approximation assumes that the molecule has a fixed bond length and it does not vibrate while it undergoes rotation about its centre of mass. This approximation makes it easy to calculate the bond length by simply knowing the rotational energy spacings of the molecule observed in a spectroscopic experiment.
The rotational kinetic energy of an object depends on its moment of inertia, I,
where is the mass of the atom , is the distance of that atom from the centre of mass of the molecule. Any molecule has three perpendicular axes of rotation each of which intersect at the centre of mass for that molecule, the moment of inertia is the rotational motion found at the center of mass of a molecule. Each of these axes of rotation could be distant from one another, or they could all be equal which leads to different types of rigid rotors. A large moment of inertia is basically based on either larger atomic masses and/or longer bonds.
Types of Rigid Rotor
[edit | edit source]| Type | Moments of Inertia | Examples |
|---|---|---|
| Linear Rotors | N2, CO2, C2H2 | |
| Spherical Rotors | CH4, SF6 | |
| Symmetric Rotors | NH3, CH3Cl | |
| Asymmetric Rotors | H2O, H2CO, Most Molecules |
Linear Rotors
[edit | edit source]In this course we are mostly concerned with linear rotors, using these systems simplifies the math. If we are using a linear rigid rotor, the bond length is constant due to the rigid rotor approximation, and the centre of mass is constant, therefore the moment of inertia can be simplifies using the reduced mass as follows:
here, is the reduced mass of the linear rotor where , and is the bond length of the diatomic linear rotor. The reduced mass the inertial mass of two atom system following Newtonian Mechanics. This can then be used to solve the Schrödinger equation which is given in example 2 below. By solving this equation we find that there are two quantum numbers which determine the energy levels of a rotating rigid rotor, M and J both of which are described below in example 2.
Zero Point Energy of Linear Rotors
[edit | edit source]Unlike the particles in a box model, which has a non zero energy minima due to the fact that the lowest energy state corresponds to the n=1 state, the linear rotor can have an energy of zero in the ground state corresponding to a molecule which is not rotating in space. This is the case since when the energy of the system is equal to zero. Since the orientation in space is not known for a linear rotor it obeys the Heisenberg uncertainty principle which states that we cannot simultaneously determine the momentum and the position of the particle. Where each energy level can exist using following equation
where J is the quantum number, is the reduced mass, re2 is the length of the bond from the center of mass and ħ is reduced Planck's constant where Planck's constant is divided by 2π. From inserting J=0 in this equation above, we see .
Degeneracy of Rotational States
[edit | edit source]The rotational levels of a diatomic only depend on the quantum number J (orbital angular momentum quantum number) and does not have any dependence on the quantum number M. However, as the quantum number J increases, the degeneracy of the states increases since there are more possible values of M, which is the magnetic quantum number which are as follows
Therefore, as the quantum number J increases, the degeneracy of the states also increases. The degeneracy of the states can be calculated using the following equation:
Degeneracy occurs when there are accessible states present at the same energy level, for example, when J=1, MJ= -1,0,+1 meaning there are 3 different degeneracies at quantum number J=1
Rotational Spectroscopy of Linear Rotors
[edit | edit source]Linear rotors which contain a permanent dipole are able to be observed through rotational-vibrational spectroscopy. These molecules exhibit fine structure of lines which are all separated by a constant value. This value can be used to determine the bond length of the diatomic in question but using the following equations.
- and
where is the spacing of the rotational lines, is the rotational constant, is planks constant which has a value of , is the speed of light and is the moment of inertia. Once we know the value of we can then use this value to calculate the bond length of the diatomic which is being observed.
Example 1
[edit | edit source]Calculation of the bond length of HCl from the rotational constant.
Rotational Angular Momentum and the Rigid Rotor Approximation
Rotational energy is purely kinetic, which is a result of angular momentum in the molecule (How quickly the molecule is spinning in space). It can be assumed that the molecule is rigid for an ideal gas, this is because the change in the bond length for a vibration is small in comparison to the total length of the bond, this is called the Rigid Rotor Approximation.
Linear Rotors
The rotation of an object depends on its moment of inertia where is the mass of atom i, and is the distance of atom i to the axis of rotation. This expression works well if the diatomic molecule consists of only the same element (e.g. ). For diatomic molecules which do not consist of the same elements (e.g. ) this expression can be more work intensive then needed. For diatomic molecules which do not comprise of the same elements the expression is significantly less work intensive to use in comparison to the previously stated equation. Where is the bond length of the diatomic molecule and is the reduced mass of the molecule, is given by the equation where is the atomic mass of atom 1, and is the atomic mass of atom 2.
Rotational Spacings

The spacings of rotational transitions are constant, and are commonly referred to as a rotational constants . For linear rotors the spacing is between each rotational transition as can be seen in the image to the right.
Utilizing the equation for the rotational constant we can find the value for and then use it in the equation and solve for the moment of inertia , where is Planck's constant, and is the speed of light. Once the moment of inertia is calculated it is simply the matter of solving for the diatomic bond length which can be found by .
Sample Problem
Calculate the Bond Length of HCl from the Rotational Constant.
Knowing HCl has a rotational constant value of 10.59341 cm−1, the Planck's constant is 6.626 × 10−34J s, and the speed of light being 2.998 × 1010 cm s−1. We then solve for the moment of inertia such that:
The next step is to calculate the reduced mass . Knowing that the mass of hydrogen is 1.00794 u, and the mass of chlorine is 35.4527 u.
Now knowing all of the variable it is just a matter of solving for the bond length .
Å
Å
Example 2
[edit | edit source]Calculation of the energy for the transition of N2 from the ground to first excited rotational state.
Solution
[edit | edit source]For N2, the reduced mass is calculated using an atomic mass of 14.0067 u for nitrogen. Since it is a diatomic molecule, the equation can be simplified to the following where represents the mass of a nitrogen atom:
For the rigid rotor, the lowest possible energy state is J=0 so the ground state rotational energy is always zero. The rotational energy levels are only dependent on quantum number J and only the ∆𝐽 = ±1 transitions can occur. In the case of the transition from the ground to the first excited state, the following is a diagram that represents the transition.

The energy for the transition J=1 ← J=0 is calculated as follows:
Vibrational energy
Molecular vibrations occur when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational and vibrational motion. Vibrational energy is different from translational energy (how a molecule translates through 3-dimensional space along the x,y, and z Cartesian coordinates) and rotational energy (how fast a molecule spins) in that the focus is placed on how a chemical bond behaves with respect to the quantum harmonic oscillator approximation.
Vibrational Energy Levels
[edit | edit source]
The Potential Energy Surface of Molecules
[edit | edit source]Chemical bonds are formed due to electrostatic interactions between protons and electrons, and can occur as nuclear-electron attractions, electron-electron repulsion, and inter-nuclear repulsion. As a bond forms due to the nuclear electron attractions between a proton and electron, the electron density increases in the space between two nuclei. For a diatomic, the potential energy is only a function of the distance between the two atoms, and the strength of these components changes depending on the distance between the atoms. Bonding is generally stronger when two nuclei are close, but this close proximity also increases internuclear and core-core repulsions. Hence, the potential energy is lowest at the equilibrium bond length . Short distances lead to high repulsion, long distances result in no interactions, and middle distances result in stabilized bonding.
Application of Hooke's Law
[edit | edit source]Molecules will have some amount of kinetic energy along this degree of freedom and harmonic oscillation will occur along the bond length. A bond between molecules can be approximated as a parabola centred around the equilibrium bond length . Hooke's Law treats the bond as a spring. When a bond is stretched or compressed its potential energy increases, just like the behaviour of a spring under the harmonic oscillation approximation. is the constant corresponding to the stiffness of the spring, called the spring constant, and can be correlated to the stiffness of a molecular bond. Higher spring constants correlate to stiffer (stronger) bonds. For example, the value of will be larger for a double bond than in a single bond. Hence, a double bond is more stiff.
We can approximate the bond as a parabola centred around the equilibrium bond length :
Quantum Harmonic Oscillator Approximation
[edit | edit source]
There are several significant approximations associated with describing chemical bonds as harmonic oscillators:
- The bond can never dissociate. In reality if we keep pulling two atoms apart the bond will eventually break, but according to the quantum harmonic oscillator approximation the potential energy just continues to increase and bonds are not allowed to break. This can be referred to as anharmonic effects; if bonds were really harmonic no chemical reaction would ever take place.
- The repulsive wall isn't repulsive enough. In reality, short distances are dominated by repulsive interactions. The low potential energy structures of most important molecules are near the minimum energy geometry.
- The spacing of the vibrational energy levels are exactly equal. However, in reality they become slightly smaller as quantum number increases.
Energy levels of a quantum harmonic oscillator follow:
where is Planck's Constant (6.626×10−34 J s), is the vibrational frequency of the oscillator in s-1 and quantum number = 0,1,2...
is related to the spring constant, , and reduced mass of a diatomic,.
Other Important Formulae
[edit | edit source]Since Planck's constant, , and vibrational frequency, , are related by , the relation can also be expressed as:
Wavenumbers, also called vibrational frequency, are reported in reciprocal distance and chemists typically use reciprocal centimetres.
where is Energy in Joules, is the speed of light (2.9979×108 m/s = 2.9979×1010 cm/s) and, is wavelength. This molecular transition energy is usually extremely small and typically follows the order of 1×10−20 joules, J.
Vibrational Zero Point Energy
[edit | edit source]The ground state does not have zero energy. This is the vibrational zero-point energy, and is consistent with the Heisenberg Uncertainty principle. If a molecule had zero vibrational energy it would be at rest at the minimum, and therefore we would know its position and momentum completely. Even in the lowest energy state, molecules still vibrate.
Vibrational Absorption Spectra
[edit | edit source]Vibrational transitions follow the selection rule
The vibrational spectrum of diatomics is coupled with rotational transitions.
The total selection rule is
For non-linear molecules:
Trends in Vibrational Frequencies
[edit | edit source]- Strong bonds are stiff bonds, which have a large spring constant ().
- Stiff bonds have large vibrational energy level spacings.
- Light atoms give small reduced masses ().
- Molecules with small reduced masses have large vibrational energy level spacings.
Vibrational Energy Example 1
[edit | edit source]The harmonic vibrational frequency of HF in wavenumbers is 4141.3 cm-1. [1]
Using this information, calculate the energy for the transition of HF from the ground to first excited vibrational state.
Solution
[edit | edit source]Vibrational energy levels of a quantum harmonic oscillator follow:
However, this relationship requires the vibrational frequency to be in units of s-1. To do this, we multiply the given value of 4141.3 cm-1 by the speed of light, in centimetres.
Now that we have the correct units, the ground state vibrational energy can be calculated as follows:
Next, the first excited state vibrational energy is:
Finally, the energy for the transition from the ground state (n=0) to the first excited state (n=1) level is:
Vibrational Energy Example 2
[edit | edit source]Calculation of the vibrational wavenumber of CO from the force constant and reduced mass.
Harmonic Oscillator Approximation
[edit | edit source]As there is some kinetic energy along the vibrational degree of freedom, the bond length undergoes oscillation. The Schrӧdinger equation for the movement of quantum mechanical particles given the potential energy function V(r) is complicated to solve. Instead, the Harmonic Oscillator Approximation is used which proposes that the bond behaves like a spring governed by Hooke's law.
Hooke's law:
where r is the internuclear distance and k is a constant corresponding to the stiffness of the spring
Given this approximation, the following relationship arises
where,
- is the force constant of the bond in
- is the reduced mass in kilograms
- is the vibrational frequency of the oscillator in
The reduced mass for a heteronuclear diatomic can be calculated as follows
- where and corresponds to the masses of each atom.
Solution
[edit | edit source]Firstly, the reduced mass of the carbon monoxide molecule must be calculated given that the atomic masses of carbon and oxygen is and respectively.
Convert into units of kilogram as follows
The next step is to derive the vibrational frequency in given the value of the reduced mass, , and the force constant [2]. Note that
Convert the vibrational frequency into wavenumbers given the following relationship
References
[edit | edit source]- ↑ Atkins, Peter and DePaula, Julio. "Elements of Physical Chemistry". Oxford University Press, 2013, p. 501.
- ↑ HyperPhysics.Quantum Physics: Vibrational Spectra of Diatomic Molecules. http://hyperphysics.phy-astr.gsu.edu/hbase/molecule/vibspe.html (accessed Feb. 2, 2016)
Electronic energy
Atoms and molecules can hold different energy levels that correspond to different distributions in how the electron density is distributed around them. These are counted as electronic degrees of freedom.
For example, the simplest atom is the hydrogen atom, with 1 proton and 1 electron. The attraction between the proton and electron in the hydrogen atom is governed by Coulomb's Law,
| principal quantum number | n=1,2,3,... |
| angular momentum quantum number | |
| magnetic quantum number | |
| spin quantum number |
Where is the elementary charge of an electron, is the distance between the proton and electron in the hydrogen atom, and is the vacuum permittivity constant. When the Schrödinger equation is solved for this potential, four quantum numbers result, n, l, ml, and ms. This means that the particles can only take on certain discrete values of energy. The electronic energy of the hydrogen atom depends only on the principal quantum number via the following equation,
Where is the mass of an electron. This means that all sets of angular momentum, l, and magnetic quantum numbers, ml, and ms, with the same value of n are degenerate, as they do not appear in this solution to the Schrödinger equation. This quantization of allowed energies give rise to the electronic energy levels of the hydrogen atom. This equation can also be used to calculate the energy levels of hydrogen like species such as He+. An atomic factor, , would be added in the numerator to account for the extra protons in the atom, which would pull the electrons in more than the hydrogen atom, and thereby increase the energy.
Example
[edit | edit source]Calculation of the energy for the excitation of O2 from the triplet to singlet electronic state.
Singlet oxygen comes from the electronic excitation of triple oxygen, the energy is experimentally 0.98 electron volts per mole of electrons. The figure below illustrates the different states of oxygen. 1Δg and 1Σg+ represents singlet oxygen as it has a spin state equal to one, the triplet degenerate state is represented by 3Σg-, the transition is represented by 1Δg←3Σg-

The degeneracy is calculated for this state is calculated from the formula gel= 2S+1 where S is the sum of the spin of the electrons. For singlet oxygen it has one spin state of -1/2 and a spin state of +1/2 giving the sum to be 0 and the degeneracy to be calculated by gel= 2(0)+1 to give a degeneracy of 1. Triple oxygen has two electrons in the spin up state to give it a value of 1. Which gives the degeneracy of 3 for this state.
The experimental value for this transition is experimentally calculated to be 0.98 eV found on Singlet Oxygen. This is for a wavelength of 1270 nm. the conversion factor from eV (electron volts) to kJ/mol is given by multiplying the number of electron volts by 96 kJ/mol, for the transition of triplet degenerate state to the singlet energy state it gives a value of 94.56 kJ/mol.
Raman spectroscopy
The Raman Effect
[edit | edit source]
The Raman effect is a form of light scattering by a material. It can be observed when a sample is exposed to an intense, high energy, monochromatic light source, such as a laser. Most photons of this light that are scattered by the material will be scattered elastically, meaning that there will be no change in the frequency of light. This results in an intense line at the same frequency of the source, known as the Rayleigh line.
Photons can also undergo Raman scattering, where they are inelastically scattered. This means that they will emerge at a higher or lower frequency. This type of scattering is less common than elastic scattering, with only 1 in 107 photons experiencing Raman scattering. Stokes lines occur when the scattered light emerges at a lower frequency. Anti-stokes lines occur when the scattered light emerges at a higher frequency.
The Raman effect is applicable even when the frequency of radiation does not correspond to a transition between molecular energy levels. This cannot be explained by standard absorption or emission and therefore must be the Raman effect.
Raman Spectroscopy
[edit | edit source]Raman spectroscopy is a spectroscopic technique which measures inelastic scattering. The gain or loss of energy by incident light corresponds to transitions between the energy levels of the molecules. These transitions are both rotational and vibrational. A high energy photon excites a molecule to a "virtual state" (v1), following this the molecule returns to a different state (v2) on emission. A different frequency of light is emitted due to the difference in initial and final energy levels.
The Gross Selection Rule for Raman Spectroscopy
[edit | edit source]The gross selection rule for Raman spectroscopy states that a molecule must be anisotropically polarizable to have a Raman spectrum. Anisotropic indicates that something is different in some directions, in this case the electron density of the molecule must be polarized heterogeneously allowing for it to be observed via Raman spectroscopy.

This selection rule explains why Raman spectroscopy can successfully analyze homonuclear diatomics, such as H2 and N2, when rotational and vibrational spectroscopic techniques cannot.
The Raman effect is present for any molecule that can be polarized anisotropically. Molecules that are spherical tops cannot be anisotropically polarized, this is because they have three equal moments of inertia. Therefore these molecules cannot be observed using Raman spectroscopy.
The Specific Selection Rule of Rotational Raman Spectroscopy
[edit | edit source]The specific selection rule for Raman spectroscopy of linear molecules is . The Raman spectrum has regular spacing of lines, as seen previously in absorption spectra, but separation between the lines is doubled. The evenly spaced lines have a separation of: where:
The rotational energy of the molecule can be calculated using the following equation:
The Specific Selection Rule of Vibrational Raman Spectroscopy
[edit | edit source]The specific selection rule for vibrational Raman spectroscopy states that only Δv = ±1 transitions are allowed. This is the same as for vibrational absorption spectroscopy. Vibrational Raman transitions occur simultaneously with rotational Raman transitions, this results in branching caused by rotational transitions in the Δv = ±1 peaks. The vibrational transitions result in 3 branches with fine structure from rotational transitions. In the spectrum each line corresponds to a change in quantum numbers v, J, or both.
| Branch | Energy Level Transition |
|---|---|
| O | ΔJ=-2 |
| Q | ΔJ=0 |
| S | ΔJ=2 |
Raman Spectroscopy Example 1
[edit | edit source]Calculation of rotational energy levels of N2 from pure rotational Raman spectrum.
For a molecule to have a Raman spectrum it must be anisotropically polarizable and thus have a different electron cloud distortion along different axis. Given the Pure Rotational Raman Spectrum of a molecule it is possible to calculate the rotational energy levels based on the spectrum itself. Transitions of can be observed in the spectrum and used to find the energy levels.
A sample calculation to find the rotational energy levels of Nitrogen can be computed using the spacings and J values of the Raman spectrum below. This calculation will be done in three steps:

1. Finding the rotational constant, .
2. Finding the moment of Inertia, .
3. Finding the rotational energy, .
Step 1: Computing the rotational constant B:
As seen from the figure above, the spacing, or v, is approximately 8.00 cm-1 which corresponds to the quantum number J = 1. Using these values it is possible to compute B by re-arranging the formula below:
Step 2: Compute the moment of Inertia I using the formula below and the constant B determined above:
Note: h is Planck's constant and c is the speed of light in cm/s: . When using frequencies in units of wavenumber (), the speed of light in units of cm/s should be used in order for them to cancel out during the calculation.
Step 3: Compute the rotational energy level using the value of I from above and the following two equations:
Note: Substituting the equation for I into the energy equation will give:
Note: Since the calculated value of was done using , this corresponds to the energy of the energy level. This calculation can be repeated using different values of to determine the different energies of each rotational energy level.
Postulates of Statistical Thermodynamics
Postulates of Statistical Thermodynamics
[edit | edit source]Statistical thermodynamics is a branch of science which utilizes statistics in order to relate the microscopic properties of a system to the macroscopic properties. Classical thermodynamics describes macroscopic properties of a system composed of atoms or molecules such as pressure, enthalpy and internal energy. On the other hand, quantum mechanics utilizes quantized values of microscopic properties of a system, such as rotational and vibrational movement, to calculate the energy of the system. Using two different methodologies, both thermodynamics and quantum mechanics can be utilized to evaluate similar properties of a system without any clear connection between the two fields. Statistical thermodynamics provides this connection by averaging the microscopic values across a large set of microcanonical ensembles at an instant in time to arrive at macroscopic values. These microcanonical ensembles are physical systems in which the volume, energy and total number of particles are held constant. The total number of microcanonical ensembles (𝒜), can be averaged to obtain a mean result, known as the mechanical value.
First Postulate of Statistical Thermodynamics
[edit | edit source]The First Postulate of Statistical Thermodynamics states that the time average of a mechanical variable M in the thermodynamic system of interest is equal to the ensemble average of M as the limit of 𝒜 → ∞. This law states that the average value of a mechanical variable taken from the ensembles of microstates matches the mechanical value predicted by classical thermodynamics, so long as the number of microstates 𝒜 is an exceedingly high number. The First Postulate of Statistical Thermodynamics can be extended to arrive at the Gibbs Postulate, which relates the energy of said microstates to internal energy of a system as calculated by classical thermodynamics.
Gibbs's Postulate
[edit | edit source]Gibbs's Postulate relates the internal energy (U) of a system, determined by thermodynamics, to the average ensemble energy (E), determined by statistical mechanics.
The average energy over the 𝒜 copies in the ensemble is given by the following equation.
Through the Gibbs Postulate, the average ensemble energy can be used to define the thermodynamic values of Helmholtz energy (), entropy (), pressure (), and thermodynamic potential () through the following relationships.
Second Postulate of Statistical Thermodynamics
[edit | edit source]The Second Postulate of Statistical Thermodynamics states that for an ensemble representative of an isolated system, the systems of the ensemble are distributed uniformly. All states consistent with the specified microcanonical system will occur with equal probability. This is otherwise known as the principle of equal 'a priori' probabilities. For example, in two microcanonical systems with three particles capable of occupying a quantum level , with , there is an equal probability of the first system occupying the states , as there is the second system occupying the states . Across the distribution for each system there is an indiscriminate occupation of each quantum state which is just as likely as any other.
Microcanonical ensemble
A microcanonical ensemble is a statistical ensemble which is used to represent the possible states of a mechanical system in which the total energy of the system is exactly specified. A micro-canonical ensemble is assumed to be isolated, thus the system cannot exchange energy or particles with the environment. The system, which can be composed of a solid, liquid or a gas is completely isolated from its surroundings and has constant energy (E), number of particles (N), and volume (V). This ensemble composes of an assembly of “copies” of this isolated system; the energy of each copy is the same, thus E=U, considering one copy of this system is sufficient.
Combinatorial Mathematics
[edit | edit source]Even though the energy of each copy of the system in a microcanonical ensemble is the same, these copies can exist in different quantum states. For example, consider a system composed of 2 hydrogen molecules, with the total vibrational energy of 2hυ.
The vibrational quantum numbers must add up to 2, however, there are, in fact, three ways of achieving this. Two possible configurations are where one molecule has n = 2, and the other has n = 0; and the third configuration is where both molecules have n = 1.
It can be noted that in the example above that the three configurations (also called microstates) form two macrostates (states with unique sets of quantum numbers) - {0,2} with a weight of 2 (because there are two ways of arriving at this configuration), and {1,1} with a weight of one. The weights of these states play an important role in Statistical Mechanics, so it is important to know a way to quickly determine them, rather than just counting them directly.
Let's consider the weights of configurations {1,2}, {1,2,3} and {1,2,3,4}. Counting the weights gives 2, 6 and 24 or 2!, 3! and 4! respectively for these three states where
represents factorial of the number n. The weights of the configurations are just permutations or ways a given set of the quantum numbers can be arranged. Using combinatorial mathematics, the weight of a certain vibrational state in the example above can be found to be
where N is the total number of molecules and ni is the number of molecules in the state i.
Generalizing, the weight of a system with occupancies in an ensemble containing systems is
Example
[edit | edit source]If there are three molecules and four units of vibrational energy, what are the possible macrostates of this system and what is the weight of each macrostate?
A number of microstates in the same system can have the same composition. These systems can be weighted depending on the occupancies of the systems. The weight of a macrostate can be defined by the equation:
Where, represents the total number of systems in the ensemble and represents the product of the factorial of the occupancies of each level.
For three molecules with four units of vibrational energy, we have four unique ways to distribute the four units of vibrational energy between the three molecules, {0,0,4}, {0,1,3}, {1,1,2} and {0,2,2,}. So for this example, = 4 and the weights of each variation of the system would then be:
References
[edit | edit source]University of Delaware.http://www.physics.udel.edu/~glyde/PHYS813/Lectures/chapter_6.pdf (accessed Feb 24, 2017).
Balakrishnan, V. The Microcanonical Ensemble, 2017.
Boltzmann Distribution
Developing the Canonical Ensemble
[edit | edit source]The first step is to develop a model of how the energy is distributed; the model which will be used is a canonical ensemble which is a system at constant number of particles, volume and temperature. A canonical ensemble can be created by first taking a microcanonical ensemble of a large number of systems, , all at the same number of particles, volume and energy. The entire microcanonical ensemble is then immersed into a heat bath. The systems are then allowed to exchange energy with the heat bath until all the systems come to a thermal equilibrium. The microcanonical ensemble is the removed from the heat bath such that the systems can only exchange energy with the other systems around it. The systems will now have a distribution of all possible total energies. These energy states can then be numbered in increasing energy (i.e., lowest energy is ). Since each of these energy states can have any number of systems with that energy, then the number of systems with total energy can be defined as the occupation of the state with variable . The entire ensemble can then be defined as the occupancies of the energy states, where the total number of systems, , is:
and the total energy of the entire ensemble, or energy level, , is:
The Weight of a Configuration
[edit | edit source]The occupancy of any of these energy levels depends on how many possible ways the total energy of the ensemble can be distributed amongst the systems or can be described by the occupancies of the energy states. Some of these configurations of the occupancies are mathematically more likely than others.
The weight of a configuration, or the number of ways to distribute the energy of the ensemble, is important. By combinatorial mathematics the weight of a system with occupancies in an ensemble containing systems is:
System Weighting
[edit | edit source]Consider an ensemble of systems divided into two states. The occupancy of the first state is N and therefore the occupancy of the second state is A-N. The weight of this configuration as a function of N is:
It can be shown that as increases the most probable distribution (N= /2) becomes much more heavily weighted than any other configuration.
The Most Probable Distribution
[edit | edit source]To describe a canonical ensemble, only the most probable set of occupancies need to be determined, denoted as a*. For a very large , the configuration with the largest weight will be much more heavily weighted than the other configurations. Therefore, this set of occupancies will be the most probable, and any configuration with a significantly large weight will be close to a*.
However, for any given ensemble, not all occupancies are possible. This is due to the imposition of constraints upon the occupancies by the specific ensemble. In a canonical ensemble there are two constraints which were previously used to define a canonical system:
- The sum over all energy levels has to add up to the total energy of the ensemble, denoted as :
- The sum of all of the occupancies must be equal to : Therefore, given these two constraints, we must find a* that agrees with the above imposed constraints of the canonical ensemble.
Calculating the Most Probable Occupancy
[edit | edit source]To calculate the most probable occupancy, we must calculate the occupancies that would give the maximum weight. Lagrange multipliers are used to maximize this function.
Mathematical Manipulation of W
[edit | edit source]It was previously given that However, this equation is difficult to manipulate. But, for a positive function, the maximum occurs at the same location as it does in the natural logarithm of the function. Since the maximum is what we’re really interested in finding, we can use to find the maximum, which makes the mathematics easier to manipulate. Therefore the equation becomes:
Applying the Math to a Canonical Ensemble
[edit | edit source]Using the Lagrange multipliers as previously described, we can determine the most probable occupancy of the canonical system. When Lagrange multipliers are applied to the equation, following the aforementioned constraints, the new equation becomes:
Next, we must simplify each of the three terms in the equation and solve for the unknown constants $\alpha$ and $\beta$.
Term 1
Using Stirling’s Approximation:
Term 2
For , there will be one term of the sum where i = j, everything else is equal to zero. Therefore, this term becomes:
Term 3
Like term 2, for , there will be one term of the sum where i = j, everything else is equal to zero. Therefore, this term becomes:
Now that the three terms have been simplified, we can combine them into one equation to solve. This gives us:
Solving for gives:
Determining and
[edit | edit source]The next step of the derivation is to determine the constants and .
We can use one of the constraints to determine .
Using this equation and the constraint, we can define the probability of a system in the ensemble as the occupation of state i divided by the total number of systems in the ensemble, which can be represented by the following equation.
Determining requires connecting it to classical thermodynamics. We can determine the average of a variable (<M>) over the states of a system by employing the equation
The average energy is
The average pressure is
Knowing these equations and combining two other equations we can come up with the following equation, which can be compared to a classical thermodynamic equation shown below.
In order to better compare the equations, we can perform a calculation such that the signs in front of and T are the same, giving the following equations:
Since multiplying through by a constant would not change the equations, we can determine that is only proportional to .
We can introduce as a proportionality constant to convert the proportionality to an equation as follows:
The Boltzmann Distribution
[edit | edit source]Earlier we determined the probability of a state within a system. Now that we know and , we can complete the equation to determine the Boltzmann Distribution.
Example
[edit | edit source]Consider a system with two singly-degenerate energy levels separated by J. Derive an equation for the probability of the system being in the ground state. Plot the probability of the system being in the ground state between temperatures 1 K and 1000 K.
is the Boltzmann constant in joules per Kelvin. The ground state has an energy level of zero (). Since the ground energy level is known and the difference between the ground energy level and the first high energy level is given (∆E = J), the high energy level can be determined using the following equation:
After using the actual values, the equation should look like this
The probability equation can then be simplified to the following:
After substituting the values determined into the equation, the probability equation should be the following:
Since the , the equation can be simplified to
Note: The probability does not have units.
This equation can be used to determine the probability of the system as the temperature changes in Kelvin.


The probability of the system, which starts from one, gradually decreases as the temperature increases. the reason why can be observed from the equation. Since the temperature is in the denominator, the probability should be smaller as the denominator increases. After about 300 k, the line pattern starts to have a slight decrease toward 0.5 of probability. In other words, the probability of Boltzmann distribution at this system becomes less probable as the temperature exceeds 1000 K.
Molecular partition functions
The partition function of a system, Q, provides the tools to calculate the probability of a system occupying state i .Partition function depends on composition,volume and number of particle. Larger the partition function allows to have more accessible energy states at that temperature.The general form of a partition function is a sum over the states of the system,
Two equivalent ways could be used to write the the partition function. Sum over states approach allows to give different indices to the states with the same energy. Energy levels approach suggests that only energy levels with distinct energies have their own index.
This requires that the energies levels of the entire system must be known and the calculations have to be calculated from sums over states. This limits the types of the systems that we can derive properties for.
For ideal gases, we assume that the energy states of molecules are independent of those in other molecules. The molecular partition function, q, is defined as the sum over the states of an individual molecule.
The particles of an ideal gas could be considered distinguishable if they are different from each other and, therefore, the unique label could be assigned to each.On the contrary, the indistinguishable particles are impossible to assign a unique label, as they are the identical to each other.This article considers the indistinguishable particles of an ideal gas for which the partition function of the system (Q) could be expressed in terms of the molecular partition function (q),and the number of particles in the system (N).As the partition function allows to calculate the probability of the system occupying the state j.Such system could be assumed as isolated, as a microcanonical ensemble of particles, where the total volume, the total energy and number of particles are constant. However, the range of energies appropriate for composition, volume and temperature of canonical ensembles contributing to the system must be still considered for composition, volume and temperature.
Partition function of an ideal gas of indistinguishable particles
Molecular Partition Functions
[edit | edit source]The energy levels of a molecule can be approximated as the sum of energies in the various degrees of freedom of the molecule,
Correspondingly, we can divide molecular partition function (q),
Translational Partition Function
[edit | edit source]The translational partition function, qtrans, is the sum of all possible translational energy states, which could be represented using one,two and three dimensional models for a particle in the box equation, depending on the system of the coordinates .The one and two dimensionsal spaces for a particle in the box equation forms are less commonly used than the three dimensional form as those do not account for the force acting on the particles inside of a box. For a molecule in the three dimensional space, the energy term in the general partition function equation is replaced with the particle in a 3D box equation. All molecules have three translational degrees of freedom, one for each axis the molecule can move along in three dimensional space.
Particle in a 3D Box Equation
The assumption that energy levels are continuous is valid because the space between energy levels is extremely small, resulting in minimal error. This form is convenient as it does not include a sum to infinity and can therefore be solved with relative ease. The translational partition function can be simplified further by defining a DeBroglie wavelength, , of a molecule at a given temperature.
Thermal de Broglie Wavelength
[edit | edit source]Rotational Partition Function
[edit | edit source]The rotational partition function, , is the sum of all possible rotational energy levels. This sum is found by substituting the equation for the energy levels of a linear rigid rotor:
Into the partition function, to produce an open form of the rotational partition function:
By solving this sum using a definite integral from zero to infinity, a closed form of this function can be found, making numerical evaluation much easier:
The full derivation of the closed form of the rotational partition function of a linear rotor is given here.
The special case of rotational partition function for homonuclear diatomics could cause the lower weight for alternating states,and result in the change of the rotational partition function. This case is based on the assumption that the total wavefunction of the two exchanging nuclei must be either asymmetric for the nuclei with the even spins (integer spins),or symmetric for the nuclei with odd spins. This function applies only to heteronuclear diatomic molecules. However, this equation can be altered by adding a variable to alter the equation based on the nature of the diatomic molecule being studied:
where is 1 for heteronuclear diatomics, and 2 for homonuclear diatomics.
The characteristic temperature is the constant that combines many constants calculated for partition functions of rotational and vibrational levels,introducing the fact of partition function dependence on the temperature change. The characteristic temperature is now defined as , where,
determining the rotational partition function can be calculated much easier using,
Vibrational Partition Function
[edit | edit source]A Partition Function (Q) is the denominator of the probability equation. It corresponds to the number of accessible states in a given molecule. Q represents the partition function for the entire system, which is broken down and calculated from each individual partition function of each molecule in the system. These individual partition functions are denoted by q. All molecules have four different types of partition functions: translational, rotational, vibrational, and electronic. Looking only at the vibrational aspect of the system, there is a specific unique equation used to calculate its partition function:
The vibrational partition function of a linear molecule is,
In general, the molecule partition function can be written as an infinite sum. This is called the open form of the equation:
It is much easier and more convenient to write this as a closed sum. This turns the equation into an approximative algebraic expression, with the following parameters in variables:
- degeneracy
- quantum number
- energy levels
The energy levels, are defined relative to the ground state of the system (i.e., the zero point energy is subtracted from each level),
By exploring some substitutions and derivations, the equation listed above for a linear molecule is achieved. The substitutions made include:
At the same time, it is important to note that represents the vibrational frequency of the molecule. It can be calculated on its own prior by the following relation:
where k represents the spring constant of the molecule and μ represents the reduced mass of the same molecule.
Electronic Partition Function
[edit | edit source]The electronic partition function (qel) of a system describes the electronic states of the system at thermodynamic equilibrium. This can be written as a sum over states,
however due to high energy levels being present under most circumstances, the electronic partition function can be reduced to:
Thus, the electronic partition function can usually be approximated as the ground state degeneracy of the atom or molecule.
Molecular Partition Function
[edit | edit source]The molecular partition function, q, is the total number of states accessible to the atom or molecule. It is the product of the vibrational, rotational and translational partition functions:
Sample problems
Problem 1
[edit | edit source]Calculate the probability of a molecule of N2 being in the ground vibrational state at 298 K.
The probability that a system occupies a given state at an instant of time and a specific temperature is given by the Boltzmann distribution.
where:
- i is the energy of the specific state, i, of interest
- kB is Boltzmann's constant, which equals JK-1
- T is the temperature in Kelvin
The denominator of this function is known as the partition function, Q, which corresponds to the total number of accessible states of the molecule.
The closed form of the molecular vibrational partition function is given by:
where:
- is the fundamental vibrational frequency of N2 in s-1
- h is Planck's constant, which is Js
This is equivalent to Q since only the vibrational energy states are of interest and there is only one molecule of N2. The equation for determining the partition function Q, from molecular partition functions, q, is given by:
where:
- N is the number of molecules
The fundamental vibrational frequency of N2 in wavenumbers, , is 2358.6cm-1 [1]
The fundamental vibrational frequency in s-1 is given by:
where
- c is the speed of light, which is cm/s
For N2,
= (2358.6cm-1) \times (2.9979 \times 10^{10| cm/s) = 7.0708 \times 10^{13}</math>
For N2 at 298 K,
The vibrational energy levels follow that of a quantum mechanical harmonic oscillator. The energy levels are represented by:
where:
- n is the quantum vibrational number, which equals 0, 1, 2,...
For the ground state (n=0), the energy becomes:
Since the vibrational zero point energy is not zero, the energy levels are defined relative to the n=0 level. This is used in the molecular partition function above and therefore, the ground state is regarded as having zero energy.
For N2 the probability of being in the ground state at 298K is:
This means that at room temperature, the probability of a molecule of N2 being in the ground vibrational state is 99.9988667%.
References
[edit | edit source]- ↑ Lide, D. R., (84th ed.). (2003-2004). Handbook of Chemistry and Physics. pg.9-85.
Problem 2
[edit | edit source]Derive an equation for the population of rotational state i of a linear diatomic. Make a bar graph of the distribution of rotational states of N2 at 298 K.
1. Equation for the population of rotational state i
For a diatomic molecule, it can be approximated as a rigid rotor. Solving Schrödinger equation of rigid rotors gives the energy levels of the molecule at state J:
where is the quantum number for total rotational angular momentum; is the rotational constant in cm-1.
- ,
where is Planck constant; is the vacuum light speed in cm/s; and is the moment of inertia.
- ,
where is the reduced mass and is the bond length.
By the Maxwell–Boltzmann_distribution, the population of rotational state i comparing to ground state is:
where is the degeneracy of the state; is the energy of the state; is Boltzmann constant and is the temperature.
Substitute ,, and the degeneracy of the state i to the equation, get:
2. Distribution of rotational states of N2
[edit | edit source]For N2, the population of state i is:
Combine the constant part, define constant .
The reduced mass μ of Nitrogen is 7.00D a=1.16×10-26 kg and the bond length r of N2 is 110 pm = 1.10×10-10m.
Substitute T = 298 K,
By sum over states, qrot=1/a = 104, so the probability of N2 occupying the ground vibrational state at 298 K is 1/104 = 9.60×10-3
The bar graph of the distribution of rotational states of N2 at 298 K is:

Problem 3
[edit | edit source]Estimate the number of translational states that are available to a molecule of N2 in 1 m3 container at 298 K.
The equation that is used to determine translational states of the molecule of N2 at 298 K is shown below.
Where (V,T) represents the partition function for translation, represents the mass of the particle in kilograms (kg), represents Boltzmann's constant , represents the temperature in kelvins , represents Planck's constant and represents the volume in 3 dimensions in .
The steps for solving this problem is shown below:
m = amu
m =
m =
Therefore, there should be translational states for N2 at 298 K.
Problem 4
[edit | edit source]Calculate the DeBroglie wavelength, rotational temperature, and vibrational temperature of N2 and Cl2 at 298 K.
The DeBroglie Wavelength
[edit | edit source]where:
- m is the mass of the molecule.
- Boltzmann's constant kB = 1.3806488×10-23 J K-1
- Planck's constant h = 6.62606957×10-34 J s
For N2 at 298K,
For Cl2 at 298K,
Unit Conversion for the DeBroglie Wavelength:
The Rotational Temperature
[edit | edit source]where:
- Planck's constant
- μ is the reduced mass.
- re is the bond length between two atoms in a molecule
For N2,
- re = 1.09769Å = 1.09769×10-10m [1]
For Cl2,
- re = 1.988Å = 1.988×10-10m [2]
Unit Conversion for the Rotational Temperature:
The Vibrational Temperature
[edit | edit source]where:
- the vibrational frequency of the molecule in wavenumbers.
- The speed of light c = 2.99792458×1010cm s-1
For N2,
For Cl2,
References
[edit | edit source]| Molecule | N2 | Cl2 |
|---|---|---|
| 1.91×10-11 m | 1.20×10-11m | |
| 2.87 K | 0.351 K | |
| 3.39×103 K | 805 K |
Problem 5
[edit | edit source]At what temperature would the probability for N2 being the ground vibrational state be reduced to 50%?
50% of the N2 molecules are in the ground state, and 50% are in the excited state. So, the population can be expressed in the following equation:
The value for the wavenumber value for the N2 molecule, taken from the NIST Webbook, is 2358.57 cm-1
This can be converted into the fundamental vibrational frequency by the relation,
- where:
- is fundamental wavenumber for the molecule in cm-1.
- is the speed of light, 2.998x1010 cm/s By knowing this relation, the fundamental vibrational frequency can be calculated.
- The population of a system can be expressed by the following equation:
- where:
- is the population of the ground state molecules
- is the energy at the ground state
- is the Boltzmann constant, 1.38064853 \times 10-23 J/K
- is the temperature of the system in K
- is the molecular vibrational partition function
is related by the following equation:
- where:
- h is the Planck constant, 6.626*10-34 J*s
- Knowing these relations, we can solve for the temperature of the system when the molecule is reduced to 50% ground state, which is when 50% of the molecules are in the ground state and 50% of the molecules are excited.
- Knowing the value of , we can solve the molecular vibrational partition function to obtain the exact value of T for when 50% of the molecules are in the ground state.
- Therefore, by substituting the Planck constant, fundamental vibrational frequency and the Boltzmann constant into the equation, we can obtain the temperature for when half of the molecules are in the ground state.
- The temperature at which 50% of the N2 molecules are in the ground vibrational state is 4895 K.
Derivation of thermodynamic functions and variables from partition functions
Variables of the Canonical Ensemble
[edit | edit source]The Canonical Ensemble partition function depends on variables including the composition (N), volume (V) and temperature (T) of a given system, where the above partition function equation is still valid with .
The partition function of the canonical ensemble is defined as the sum over all states of a particular system involving each states respective energies, and represented by the following equation,
In this equation, represents the Boltzmann Constant with a value of , T represents the temperature in kelvin and is the energy at state j.
Internal Energy
[edit | edit source]The partition function can also be related to all state functions from classical thermodynamics, such as U, A, G and S. The ensemble average of the internal energy in a given system is the thermodynamic equivalent to internal energy, as stated by the Gibbs postulate, and defined by,
Where the variable Q here represents the partition function term. For a canonical ensemble, the internal energy can be derived from the above equation by considering the derivative of Q with respect to T,
Rearranging this resulting equation for yields,
and from the Gibbs Postulate,
Helmholtz Energy
[edit | edit source]The Helmholtz Energy, or Helmholtz Free Energy, of a Canonical Ensemble represents the amount of work and energy obtainable by a certain closed system under constant concentration, volume and temperature. The expression for A utilizes both the internal energy and entropy of the system, and is derived by,
Where A is the Helmholtz Energy term, U represents the internal energy and S represents the entropy of the system being studied. Substituting the per-determined values for the canonical ensemble,
Q represents the partition function for this particular system, and is proportional to the absolute free energy. Thus, an increase in will occur with an increase in the number of total accessible states, and a more negative free energy.
Entropy
[edit | edit source]The Gibbs definition of Entropy is described by,
where represents the Probability of being in state i and described by the weight of the state divided by the sum of all possible weights, such as,
and thus large probabilities of multiple states correlate with large values of S. By using the above Gibbs definition, the entropy of a Canonical Ensemble (NVT) can be derived, in terms of Q, to yield the following expression,
or
The value of Q in this equation can also be represented as , where q is equivalent to the partition function of the molecules, with N representing the number of molecules, in the system.
The partition function is also represented by the denominator of the probability term for a certain state, given by the following,
Chemical potential
[edit | edit source]The Chemical Potential, μ, represents the change in the Helmholtz free energy when an additional particle is added to the canonical ensemble system. A heterogeneous system represents a system containing more than one type of gas and the expression for the chemical potential here is μi, where i represents each differing species. This term is derived from Helmholtz free energy, A, with respect to number of molecules for a certain species i. Thus,
And utilizing the above Helmholtz expression, the following can be derived,
This definition helps describe the change in the Helmholtz free energy with a changing system composition, such as a reaction forming and/or consuming certain molecules in a system.
Heat Capacity
[edit | edit source]In this section, we will complete the deviation of heat capacity in terms of the partition function Q.
According to the Gibbs postulate, the ensemble average is equal to the average internal energy, U
Substitute equation for partition function into ensemble average.
, which is the Boltzmann's Constant.
Now substituting you get,
, which is the heat capacity.
To find the equation of heat capacity using Statistical Mechanics we first have to differentiate the ensemble average with respect to .
Therefore, the equation of heat capacity with respect to the partition function Q is,
Chemical Equilibrium
Chemical Equilibrium from Statistical Thermodynamics
[edit | edit source]Consider the general gas phase chemical reaction represented by
where A, B, C and D are the reactants and products of the reaction, and is the stoichiometric coefficient of chemical A, is the stoichiometric coefficient of chemical B, and so on. Each of the gases involved in the reaction will eventually reach an equilibrium concentration when the forward and reverse reaction rates become equal. The distribution of reactants to products at the quilibrium point is represented by the equilibrium constant ():
If the system is not at equilibrium, a shift in the number of reactants and products will occur to lower the overall energy of the system. The difference in the energy of the system at this non-equilibrated point and the energy of the system at equilibrium for any particular species is termed chemical potential. When both temperature and volume are constant for both points aforementioned, the chemical potential of species i is expressed by the equation
where A is the Helmholtz energy, and is the number of molecules of species i. The Helmholtz energy can be determined as a function of the total partition function, Q:
where is the Boltzmann constant and T is the temperature of the system. The total partition function is given by
where is the molecular partition function of chemical species i. Substituting the molecular partition funCtion into the equation for helholtz energy yields:
and then further substituting this equation into the definition of chemical potential yields:
rearranging this equation the following derivative can be set-up:
From this point Sterling's approximation
can be substituted into the derivative to yield:
From here the derivative can be rearranged and solved:
A variable, , is then defined such that , where j = A, B, C or D and is taken to be positive for products and negative for reactants. A change in therefore corresponds to a change in the concentrations of the reactants and products. Thus, at equilibrium,

From Classical thermodynamics, the total differential of A is:
For a reaction at a fixed volume and temperature (such as in the canonical ensemble), and equal 0. Therefore,
Substituting the expanded form of chemical potential:
For the reaction :
This equation simplifies to
By dividing all terms by volume, and noting the relationship where is referred to a number density, the following equation is obtained:
Partition Functions
[edit | edit source]The molecular partition function, is defined as the product of the translational, rotational, vibration and electronic partition functions:
These components of the molecular partition function may be defined as follows:
where m is the mass of a single particle and h is Planck's constant, and T is temperature.
where is the reduced mass of the molecule, is the bond length between the atoms in the molecule, and is the reduced Planck's constant.
where is the vibrational frequency of the molecule.
where is the degeneracy of the ground state, and is the bond energy of the molecule.
Thus, the equilibrium constant of a chemical reaction can be expressed in terms of the molecular partition functions and the difference in atomization energies of the products and reactants .
Example
[edit | edit source]Calculate the equilibrium constant for the reaction of and at 650 K.
Equilibrium Constant Equation (From Molecular Partition Functions)
A simple problem solving strategy for finding equilibrium constants via statistical mechanics is to separate the equation into the molecular partition functions of each of the reactant and product species, solve for each one, and recombine them to arrive at a final answer.
In order to simplify the calculations of molecular partition functions, the characteristic temperature of rotation () and vibration () are used. These values are constants that incorporate the physical constants found in the rotational and vibrational partition functions of the molecules. Tabulated values of and for select molecules can be found here.
| Species | (K) | (K) | (kJ mol-1) |
|---|---|---|---|
| 6125 | 0.351 | 239.0 | |
| 808 | 87.6 | 431.9 | |
| 4303 | 15.2 | 427.7 |
Combining the terms from each species, the following expression is obtained:
At 650 K, the reaction between and proceeds spontaneously towards the products. From a statistical mechanics point of view, the product molecule has more states accessible to it than the reactant species. The spontaneity of this reaction is largely due to the electronic partition function: two very strong H—Cl bonds are formed at the expense of a very strong H—H bond and a relatively weak Cl—Cl bond.
Eyring Transition State Theory
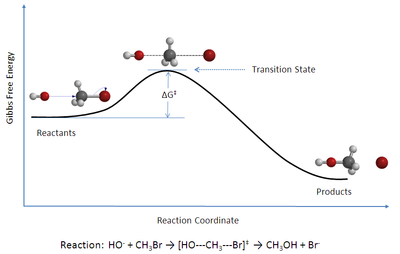
Chemical kinetics describes the rates of change of the concentration of species through chemical reactions.
- Rate=
The rate constant, k, is depends on the chemical species undergoing reaction and the reaction conditions (i.e, temperature).
Rate constants of elementary gas phase chemical reactions can be estimated by statistical thermodynamics using a rate theory. One of the simplest and more widely used theories is Eyring conventional transition state theory.
In Eyring conventional transition state theory, the rate constant of a chemical reaction can be expressed in terms of the partition functions of the transition state and the reactants. is the barrier height of the reaction.
Assumptions of Transition State Theory
[edit | edit source]Transition state theory relies on three key assumptions in its derivation.
- Reactants are in constant equilibrium with the transition state structure.
- The energy of the particles follow a Boltzmann distribution.
- Once reactants become the transition state, the transition state structure does not collapse back to the reactants.
Given a reaction where reactants A and B are converted to a transition state C:
The equilibrium constant, K‡, can be written in terms of the concentrations of the reactants and the transition state.
This reaction exists as an equilibrium because we assume that not every collision results in the formation of the transition state. Rewriting the concentrations in terms of the partition functions yields the equation below.
Thermodynamic Representation of Eyring Equation
[edit | edit source]The Eyring equation can also be expressed in terms thermodynamic parameters. The standard free energy, ΔG‡, can be written in terms of the equilibrium constant.
Using the equation for standard Gibb's energy, the Eyring equation can be written as:
Here, is the enthalpy of activation and represents the enthalpy or energetic stability of the transition state structure; it is the energy needed to break the bonds of the reactants and form the transition state. Similar to standard enthalpy, a negative value represents a more stable energetic structure and vice versa. is the entropy of activation, which represents the entropy, or disorder of the transition state and is the number of accessible states of transition state compared to the reactants. Likewise, a positive entropy represents a more disordered system while a negative entropy corresponds to a more ordered system.
Using the thermodynamical representation of the Eyring equation allows for the prediction of the type of mechanism a reaction can take. An associative mechanism involves a molecule bonding to another to form the transition state, while a dissociative mechanism involves the breaking of a molecule to form the transition state. In general, an associative mechanism occurs when the entropy of the transition state is lower than the reactants and thus the transition state is more ordered; this means change in entropy is negative. the result of this is that the entropy is lower and the structure is more ordered and has less accessible states. Contrary, a dissociative mechanism occurs when the entropy of the transition state is positive or higher for the transition state relative to the reactants. Thus, the transition state is less ordered than the initial reactants.
Partition functions of the transition state
[edit | edit source]TST requires the calculation of the partition functions for the transition state structure.
The translational and rotational partition functions of the transition state have the same form as those used for the reactant species. The vibrational partition function differs slightly because the vibration that corresponds to crossing the barrier is removed from this term (it "becomes" the term. As result, there is one fewer term in the vibrational partition function. For non-linear transition states, there will be terms. For linear transition states, there will be terms.
Calculation of a rate constant using this theory requires that all the structural and mechanical properties of the transition state be known. This is a challenge because it is very difficult to use spectroscopy to determine the structural properties of a transition state, which typically only exists for 10-200 fs. Instead, computational chemistry is commonly used to calculate the properties of transition states.
Example
[edit | edit source]Calculate the rate constant for the reaction of H + H2 at 300 K.
H + H2 → H2 + H
To calculate the rate constant the following are required, for both the reactants and the transition state:
- the masses of isotopes
- vibrational frequencies (qvib)
- moments of inertia (qrot)
- electronic degeneracies'
- reaction barrier height
| Species | Vibration | (cm-1) |
|---|---|---|
| H2 | H-H stretch | 4401.1272 |
| H-H-H‡ | H-H-H bend (degeneracy of 2) | 875.62 |
| H-H-H‡ | H-H-H symmetric stretch | 2050.67 |
R = 0.743 Å for the reactant R = 0.931 Å for the transition state ‡ = 38.61 kJ mol-1
translational partition functions:
for H2:
For H-H-H:
rotational partition functions:
for H2 :
For H-H-H:
vibrational partition functions:
for H2:
Rate constant k:
Links
[edit | edit source]References
[edit | edit source]Atkins, P., de Paula, J., Elements of Physical Chemistry, 5th ed.; Oxford University Press, 2009.
Blackbody Radiation
Blackbody radiation is the phenomenon where a heated object will emit electromagnetic radiation

Rayleigh–Jeans Law
[edit | edit source]The average thermal energy of a classical harmonic oscillator is predicted by equipartition theorem to be,
Combined with the Rayleigh model, the blackbody intensity is predicted to be,
This purely classical theory is known as the Rayleigh–Jeans Law. Although it provides a reasonable description of the intensity of blackbody radiation at low frequencies, it incorrectly predicts that the intensity will continue to increase at higher frequencies, although experiments show that the intensity should reach a maximum and then decline at high frequencies. This is known as the ultraviolet catastrophe.
Planck's Law
[edit | edit source]If we derive the average energy for the oscillator based on the quantum harmonic oscillator, this equations becomes
Planck's law states that[1]
where
- Bν(T) is the spectral radiance (the power per unit solid angle and per unit of area normal to the propagation) density of frequency ν radiation per unit frequency at thermal equilibrium at temperature T.
- h is the Planck constant;
- c is the speed of light in a vacuum;
- k_B is the Boltzmann constant;
- is the frequency of the electromagnetic radiation;
- T is the absolute temperature of the body.
Principles of the Kinetic Theory of Gases
Kinetic Theory of Gases
[edit | edit source]The kinetic theory of gases is a simple model used to describe the behavior of molecules in the gas phase. It is based on the following assumptions:
- The gas is made up of a collection of non-interacting particles separated by a distance larger than its dimensions
- Particles move in random directions such that direction of motion has a negligible effect on the properties of the gas (it is isotropic).
- All collisions are perfectly elastic. Particles may collide with the walls of the container or with each other.
- Particles carry only translational kinetic energies.
Given that translational energy spacing is relatively small compared to KT, particles behave classically and follow Newtons law's of motion. In large systems, in is unrealistic to monitor the velocity of individual particles so instead an average is taken. Of the range of possible velocities some will be more common than others, giving rise to a probability distribution, better known as the Maxwell distribution.
The probability that a particle will have a given velocity is provided by the equation below. Ω is the probability density.
Here we have assumed that the probability distributions in each direction are independent of each other, thus it is seperable.
Each particle has translational kinetic energy characterized by
The energy distribution is expected to have a form similar of that of the canonical ensemble as follows.
By analogy, the velocity distribution is
A is later found to be
In order to find the probability of having a particular velocity we must integrate Equation 1 over all space. The function must be normalized such that the probability is unity. The leading coefficient C will take care of this.
Recall that , which leads to the following.
The exponentials are of the form of the Gaussian curve and follow
In this case,
Evaluating gives
Since the gas is isotropic the function Ω must depend only on speed. The magnitude of the velocity vector is given as follows.
Up to this point, everything has been done using Cartesian coordinates. However due to the spherical symmetry of the system is it much more natural to work in spherical coordinates.
The Maxwell Distribution of Molecular Speeds
The term comes from the conversion to spherical coordinates (Integrating over the entire surface).
Example
[edit | edit source]Find the average value of and the kinetic energy associated with motion along the x-direction.
It is best to approach this problem using the Cartesian form since we the integral is simplified considering that the function is independent of y and z.
This is an even function, therefore we can integrate over one half of the function then multiply by 2.
Multiplying this result by will give a kinetic energy of . For 3 independent direction, the total kinetic energy for an ideal gas in equilibrium is .
Sources
[edit | edit source]“Kinetic Theory of Gases and the Boltzmann Equation.” Statistical Mechanics, by Donald A. McQuarrie, New York, 1976.
Engel, Thomas, et al. Physical chemistry, quantum chemistry, and spectroscopy. Pearson Education, Inc., 2019.
Effusion
Effusion of a gas from a container
[edit | edit source]Consider a container filled with a gas at a given pressure. The container is separated from another container containing no gas (i.e. a vacuum) by a partition with a small hole of surface area . The gas from the first container will travel through the hole and into the second container at a given rate. This phenomenon is called effusion and the rate at which the gas travels through the hole is called the rate of effusion.
The rate of effusion can be defined as the product of the hole's surface area, , and the rate at which the particles collide with the walls of the container, .
Rate =
Where is given by:
Derivation of the time dependancy on pressure
[edit | edit source]The rate of effusion derived below is the rate with respect to the gas escaping the filled container.
Rate =
Solving the differential by integration.
Molecular collisions
Viscosity
What is Viscosity?
[edit | edit source]The viscosity of a fluid (gas or liquid) is a measurement of the resistance said fluid has to its deformation by stress. For a liquid, this is a relatively easy concept to understand. Water, for example, has a lower viscosity than maple syrup. The concept is slightly less tangible when considering a gas. The system we will analyze in order to better understand the viscosity of a gas is two parallel plates where the gas is contained between them.
In order to establish this relationship, we must consider the bottom plate stationary. That is,
The top plate is considered to be moving with constant velocity. That is,
where is a constant velocity.
When a gas is filling the gap between the two plates, the gas molecules exert a force of drag on the motion of the upper plate. This Shear Stress is the previously mentioned force of drag per unit area between the two parallel planes. This relationship is shown by the following formula:
where is the shear stress of the gas on the motion of the upper plate. The shear stress can also be represented by the relationship:
where a is the distance between the plates and is the viscosity coefficient of the specific molecule between the plates.
Viscosity Coefficient
[edit | edit source]As previously mentioned, the viscosity coefficient is a specific constant for the gas being examined. The Kinetic Theory of Gases must be considered in order to derive the equation for the viscosity coefficient. It is assumed from this theory that:
- The particles are hard spheres which implies that elastic collisions occur when particles are within the radius of another molecule.
- The distributions of these velocities follow the Maxwell Distribution of molecular speeds.
From these requirements determined by the Kinetic Theory of Gases, the simple version of the viscosity coefficient can be derived.
This value of is derived by a simple method for the derivation of transport properties of hard spheres. A more rigorous analysis shows that the true values for the viscosity coefficients of gasses follows this formula more closely:
The only difference between these two determinations of the viscosity coefficient is the numerical factor. The ratio of these two equations is determined here:
This ratio shows that the simple derivation is essentially correct and is close enough where the viscosity coefficient of gasses is considered.
When looking at the formula for the viscosity coefficient, it is clear that the viscosity if a gas is not dependent on very many factors. The viscosity coefficient only depends on the mass of the molecule,, the collision cross section, , and the Temperature of the gas. Since the viscosity coefficient of a gas is proportional to the viscosity of the gas, the impacts of these values are outlined below:
- The viscosity of the gas increases as the mass of the molecule increases.
- The viscosity of the gas decreases as the collisional cross section of the molecule increases.
- The viscosity of the gas increases as the temperature of the molecule increases.
These general trends are the most important take away of the viscosity coefficient.
Viscosity Comparisons
[edit | edit source]We will now look at some examples of the viscosity coefficients of some molecules and determine how the trends outlined above apply. The first example we will look at is the viscosity coefficients of N2 and CO2 at 273.15 K. The value of for these molecules respectively are 16.6 and 13.7 respectively. Even though the mass of COTemplate:Sub is much higher than that of N2, the viscosity coefficient of CO2 is slightly lower than that of NTemplate:Sub. This is due to the fact that since carbon dioxide is much larger in its collision cross section, this outweighs the fact that it is heavier than nitrogen. This is most because the collision cross section is squared while the mass of the molecule is square-rooted. This concludes that the viscosity coefficient of the molecule depends more on its collision cross section than its mass.
Another example of the phenomenon outlined above is the viscosity coefficients of helium and argon. The viscosity coefficients of these molecules are 29.7 and 21.0 Pa s. Helium is much lighter than argon but it has a much larger viscosity coefficient due to its much smaller collision cross section.
One example which shows a greater dependence on the mass than the collision cross section is shown when examining the viscosity coefficients of argon and krypton. The viscosity coefficients of these molecules at 273.15 K are 21.0 and 23.3 Pa s, respectively. This is because the collision cross sections of the two atoms are relatively close so the masses begin to have a larger impact. The mass of krypton is almost double of that of argon so this drastic difference in mass combined with the relatively close collision cross sections makes it so that the viscosity of krypton is larger than the viscosity of argon.
References
[edit | edit source]
Thermal conductivity
Thermal Conductivity of a gas based on the Kinetic Theory of Gases
[edit | edit source]Kinetic Theory of Gases
[edit | edit source]
The Kinetic Theory of Gases is a set of assumptions about a system of gas particles. These assumptions consider the system in the classical sense, as opposed to a realistic or quantum system. The gas particles are considered as perfectly round spheres, with each sphere having equal mass. It is assumed that a system has a statistically sufficient number of these spheres. Each sphere is assumed to be experiencing a very large number of collusions with other spheres and the boundaries of the system. These collisions are assumed to be perfectly elastic as there is no loss of energy from either sphere upon impact. It is also assumed that each sphere is in constant, random motion within the system. The velocities of the gas particles follow the Maxwell distribution.
Transport Properties of Gases: Momentum
[edit | edit source]Using the Kinetic Theory of gases, the function relating the transport of a property through a gas can be determined. One can imagine two parallel plates along an x-axis separated by some amount a of the z-axis. The area between these plates contains many gas particles. The upper plate moves along the x-axis at some constant velocity. A drag force is acted on this movement by the gas particles between the two plates. This is due to the transfer of momentum from the plate to the gas particles. The shear stress () is defined as the drag force per unit area. The magnitude of this drag force is dependent on the velocity of the plate, the spacing between the two plates, and the type of gas particles in the system. Each gas has a constant that effects the drag force. The viscosity coefficient, defined as , is proportional to the stress the upper plate exerts.
The gas particles nearest both plates are assumed to share equal linear momentum in the x-axis to the respective plates. That is the momentum of gas particles near the plate in motion share equal linear momentum in the direction of plate movement. A function of linear momentum in relation to the height on the z-axis is defined as G(x). It is assumed as well that each gas particle at some value on the z-axis (Z) shares the same momentum as all other gas particles at Z. With this assumption, G(x) can be defined as:
Gas particles closer to the plate in motion will contain a greater momentum. These gas particles will tend to transfer their momentum to particles closer to the stationary plate. This transfer of momentum is defined as momentum flux, and can be expressed by the rate that momentum is transferred across a plane in the system per unit area, per second. The number of particles crossing a given plane within a given time is constrained by the velocity of any given gas particle to cross the plane. The distance the particle would have to travel to pass through the plane within a given time frame is directly related to the velocity of the particle. The net flux of momentum for particles at some height z’ can be determined by integrating the product of the x-momentum of particles at z’ with the number of particles crossing through a plane. By expressing the momentum of the particles in terms of z on the z-axis and substituting G(z’) in terms of G(z) by use of a Taylor series approximation the viscosity coefficient is defined as:
Transport Properties of Gases: Kinetic Energy
[edit | edit source]If the two plates described above are kept stationary but have differing temperatures, a temperature gradient will form between them. Gas particles closer to the higher temperature plate will contain more kinetic energy than the gas particles by the lower temperature plate. This is due to the particles gaining energy when they collide with the higher temperature plate. In much the same way as momentum was transferred through the gas particles, the kinetic energy is also transferred. The rate at which this occurs is measured as the flux (J) of energy through the gas. The flux for a system is equal to the temperature gradient multiplied by the viscosity coefficient (). This flux can then be multiplied by the area of the plate to yield the rate of heat transfer. By defining a new function as the average energy of a particle at height z, one can replace in the above equation to determine the thermal conductivity coefficient.
A more rigorous derivation of the thermal conductivity coefficient based on the Newtonian mechanics of the collisions of gas particles provides a more accurate equation [5]:
This equation has a different numerical prefactor than the simple derivation, but has the same dependence on the temperature and heat capacity.
Interpretation of Thermal Conductivity
[edit | edit source]The thermal conductivity coefficient for a gas is independent of its pressure. The amount of gas within a system does not affect the thermal conductivity coefficient. However, the thermal conductivity is dependent on temperature. An increase in temperature relates directly to an increase in a gases thermal conductivity. Gases with a larger radius, , have a lower thermal conductivity. This is due to the distance traveled between collisions being shorter. A gas particle with a amount of momentum or kinetic energy will travel a shorter distance towards the lower plate, in the system described above, than a gas with shorter .
Diffusion
Diffusion
[edit | edit source]Diffusion is the movement of chemicals from an area of high concentration to an area of lower concentration due to random movement of particles. Molecules diffuse so that they are spread out evenly in they space they are occupying, which can effect where a chemical reaction takes place.
Diffusion is pressure, temperature, and mass dependent. Diffusion is slower at high pressure and for heavier particles, and diffusion is faster at high temperatures.
Fick's Law
[edit | edit source]Fick's law relates the concentration gradient to the rate of diffusion. In other words, the rate of flow of a molecule is related to the concentration gradient of that species and its diffusion coefficient. The equation for the rate of diffusion in one dimension is
where D is the diffusion coefficient, and ρN is the number density of the particle.

For particular boundary conditions, Fick's law can be integrated to give the position-dependent number density of the diffusing gases at a function of time.
Diffusion of two Gases
[edit | edit source]If there are two gases in a container with different masses and velocities, the diffusion coefficients of the gases will be
where l is the mean free path length of particle N, and vN is the average velocity of particle N.
The mean free path length of two component forms is complex due to the fact that more than one type of collision can occur, and the collision cross section of each species is involved,
where σ is the collision cross section.
Combining the diffusion coefficient equation and the mean free path length equation, the diffusion coefficient for one particle becomes,
or,
Self Diffusion
[edit | edit source]The self diffusion of a gas occurs when there is only one component in the system. Self diffusion occurs constantly as a gas molecule moves around in it's container. It can be observed by isotopic labeling. Since the gas molecules are the same the equation for the diffusion constant simplifies to
or,
for the diffusion coefficient in terms of collisions.
Definition of variables
This section will contain a list of definitions of physical variables used in this book.
| Variable | Name | Definition |
|---|---|---|
| U | Internal Energy | Internal energy is the total energy needed to create a system. It can be divided into the sum of two other energies (internal kinetic energy and internal potential energy). |
| H | Enthalpy | Enthalpy is a thermodynamic variable which corresponds to the total energy of the system and the energy required to create a volume. It has units of energy and can be calculated using the equation H = U +pV |
| q | Heat | The thermal energy transferred from the surroundings to the system. |
| w | Work | The energy corresponding to the expansion of the system against the surroundings. |
| S | Entropy | Changes in the state of a system can also involve a change in the degree of disorder in the system and surroundings. The thermodynamic function entropy corresponds to this disorder. |
| A | Helmholtz Energy | The change in energy found within a canonical ensemble. This can be calculated by the following equation: A = U - T x S |
| G | Gibb's Energy | The change in energy found within an isothermal-isobaric ensemble. This can be calculated by the following equation: G = H - T x S |
| I | Inertia | The moment of inertia is what the rotation of an object depends on. |
| Q | Partition Function | The sum over all states of a system. It is used in the denominator of the Boltzmann distribution. |
| μ | Chemical Potential | For the canonical ensemble, the chemical potential, is the Helmholtz free energy change when a particle is added to the system. |
| F | Fraction of Molecules at a Given Speed | The fraction of molecules within a range of speed (F) is an integral over the probability distribution. |
| ℓ | Mean Free Path | The mean free path is the average distance that a molecule can travel before it will undergo a collision. |
| τ | Shear Stress | The drag force per unit area. |
| J | Momentum Flux | The rate that momentum passes through a plane per unit area, per unit second |
Equations for reference
Equation Sheet 1
[edit | edit source]Translational States
[edit | edit source]The translational energy of a particle in a 3 dimensional box is given by the equation:
Where h is Planck's constant , m is the mass of the particle in kg, n is the translational quantum number for the denoted direction of translation (x, y, z) and a, b, and c are the length of the box in the x, y, and z directions respectively. The translational quantum number, n, may possess any positive integer value.
Rotational States
[edit | edit source]The moment of inertia for a rigid rotor is given by the equation:
where mi is the mass an atom in the molecule and ri is the distance in meters from that atom to the molecule's center of mass. For a diatomic molecule this formula may be simplified to:
where re is the internuclear distance and μ is the reduced mass for the diatomic molecule:
In the case of a homonuclear diatomic molecule, the reduced mass, μ, can be further simplified to:
The energy of a rigid rotor occupying a rotational quantum state J (J = 0,1,2,...) is given by the equation:
where and the degeneracy of each rotational state is given by .
The frequency of radiation corresponding to the energy of rotation at a given rotational quantum state is given by:
where is the rotational constant, and can be related to the moment of inertia by the equation:
where c is the speed of light.
To avoid confusing in obtaining values for the frequency of radiation, , and the rotational constant, , c is often expressed in units of cm/s ()
Vibrational States
[edit | edit source]The energy of a simple harmonic oscillator is given by the equation:
where n = 0,1,2,... is the vibrational quantum number and ν is the fundamental frequency of vibration, given by:
where k is the bond force constant.
Electronic States
[edit | edit source]An electron in an atom may be described by four quantum numbers: the principle quantum number, ; the angular momentum quantum number, ; the magnetic quantum number, ; the spin quantum number, .
For a hydrogen-like atom (possessing only a single electron), the energy of the electron is given by the equation:
where is the mass of an electron, e is the charge of an electron, and is the permittivity of free space.
For a system with multiple electrons, the total spin for the system is given the sum:
The electronic degeneracy of the system may then be determined by
.
Thermodynamic Relations
[edit | edit source]There exist a number of equations which allow for the relation of thermodynamic variables, such that it is possible to determine values for many of these variables mathematically starting with just a few:
where H is enthalpy, U is internal energy, p is pressure, and V is volume;
where G is Gibbs energy, T is temperature and S is entropy;
where A is Helmholtz energy;
where q is heat and w is work;
where is the heat associated with a reversible process;
where n is the number of moles of a gas and R is the ideal gas constant ().
The heat capacity for a gas at constant volume may be estimated by the differential:
while pressure may be estimated by the similar calculation:
allows for the determination of q:
may also be related to the heat capacity at constant pressure:
which in turn allows for the determination of enthalpy:
Overall heat capacity in each case may be related to molar heat capacity by relating the number of moles of gas:
Finally, the internal energy contribution from translational, rotational, and vibrational energies of a gas may be determined by the equation:
where are the translational, rotational, and vibrational degrees of freedom for the molecule, respectively.
For linear molecules the internal energy simplifies to:
And for non-linear molecules:
Equation Sheet 2
[edit | edit source]Formula to calculate the internal energy is given by the formula
Where U is the internal energy of the system is the energy of the system, is the Boltzmann constant (1.3807 x 10^-23 J K-1), T is the temperature in Kelvin, and Q is the partition function of the system.
Canonical Ensemble
[edit | edit source]Internal Energy, U, of Canonical Ensemble:
Where is the Boltzmann constant, T is the temperature in Kelvin, and Q is the partition function of the system.
Entropy, S, of the Canonicial Ensemble:
Where E is the ensemble average energy of the system, is the Boltzmann constant, T is the temperature in Kelvin, and Q is the partition function of the system.
Helmholtz Free Energy, A, of the Canonical Ensemble
Where is the Boltzmann constant, T is the temperature in Kelvin, and Q is the partition function of the system.
Partition Functions
[edit | edit source]Function to calculate the partition function, Q, in a system of N identical indistinguishable particles can be calculated by:
Where q is the molecular partition functions.
Molecular Partition Function
[edit | edit source]
In which q is the molecular partition function, is the molecular partition function of the translational degree of freedom, is the molecular partition function of the rotational degree of freedom, is the molecular partition function of the vibrational degree of freedom, and is the molecular partition function of the electronic degree of freedom.
Molecular Translational Partition Function
[edit | edit source]
Where is the molecular partition function of the translational degree of freedom, is the Boltzmann constant, m is the mass of the molecule, T is the temperature in Kelvin, V is the volume of the system.
To simplify the calculation, the de Broglie wavelength, Λ, of the molecule at a given temperature may be used. The de Broglie wavelength is defined as:
This simplifies the translational molecular partition function to:
Molecular Rotational Partition Function
[edit | edit source]
Where is the molecular partition function of the rotational degree of freedom, T is the temperature in Kelvin, is the Boltzmann constant, is the bond length of the molecule, μ is the reduced mass, h is Planck's constant, is defined as , and σ is the symmetry factor (σ = 2 for homonuclear molecules and σ = 1 for heteronuclear molecules).
The constants in the rotational molecular partition function can be simplified to the characteristic temperature, Θr, which has units of Kelvin:
Using the characteristic temperature, the rotational molecular partition function is simplified to:
Molecular Vibrational Partition Function
[edit | edit source]
Where is the molecular partition function of the vibrational degree of freedom, T is the temperature in Kelvin, is the Boltzmann constant, h is Planck's constant, and υ is the vibrational frequency of the molecule defined as:
Where k is the spring constant of the molecule and μ is the reduced mass of the molecule.
The characteristic temperature Θυ may be used to simplify the constants in the molecular vibrational partition function to the following:
Using the characteristic temperature, the vibrational molecular partition function is simplified to:
Molecular Electronic Partition Function
[edit | edit source]
Where is the molecular partition function of the electronic state and g1 is the degeneracy of the ground state.
For large temperatures, the equation turns to:
Where D0 is the bond dissociation energy of the molecule, and is the Boltzmann constant.
Simplified Molecular Partition Function
[edit | edit source]All molecular partition functions combined are defined as:
Which simplifies further when utilizing the de Broglie wavelength for the translational molecular partition function and the characteristic temperatures for the rotational and vibrational molecular partition functions.
Which is equivalent to:
Chemical Equilibrium
[edit | edit source]Determination of equilibrium constant is found by the following equation:
In which qA, qB, qC, and qD are partition functions of each species and with corresponding ν and ρ values for corresponding stoichiometric coefficients and partial pressures.
The equilibrium constant in terms of pressure can be expressed as;
The chemical potential can be determined by
in which is the change in Helmholtz energy when a new particle is added to the system
The pressure, p, can then be determined by
Data
| Molecule | (K) | (K) |
|---|---|---|
| H2[6] | 87.547 | 6332.52 |
N2Invalid parameter in <ref> tag |
2.0518 | 3393.54 |
O2Invalid parameter in <ref> tag |
2.0793 | 2273.60 |
F2Invalid parameter in <ref> tag |
1.2808 | 1318.87 |
HFInvalid parameter in <ref> tag |
41.345 | 5954.27 |
HClInvalid parameter in <ref> tag |
15.240 | 4303.41 |
NOInvalid parameter in <ref> tag |
2.4524 | 2739.79 |
| C2H2[7] | 1.7012 |
Example
[edit | edit source]Calculate the ground state characteristic rotational () and characteristic vibrational () temperatures for molecular hydrogen, H2.
Where is the reduced Planck constant, is the internuclear distance for ground state hydrogen[6], is the Boltzmann constant, and is the reduced mass.
The characteristic vibrational temperature () is calculated using the following equation
Where is Planck's constant, is the Boltzmann constant, and is the vibrational frequency of the molecule. To retain units of K the vibrational frequency must be changed to units of s-1.
References
[edit | edit source]- ↑ Rybicki & Lightman 1979, p. 22
- ↑ McQuarrie, D. A., Statistical Mechanics, University Science Books, 2000, QC 174.8 M3
- ↑ McQuarrie, D. A., Statistical Mechanics, University Science Books, 2000, QC 174.8 M3
- ↑ McQuarrie, D. A., Statistical Mechanics, University Science Books, 2000, QC 174.8 M3
- ↑ Boltzmann, L. and Brush, S.G. (1964). Lectures on Gas Theory, University of California Press ISBN 0486684555
- ↑ a b http://webbook.nist.gov/cgi/cbook.cgi?ID=1333-74-0
- ↑ E. Plyler, E. Tidwell, and T. Wiggins, (1963). Rotation-Vibration Constants of Acetylene. Journal of Optical Society America. Table 4, Data section in appendix
Essential mathematics for statistical thermodynamics
Exponentials and Logarithms
[edit | edit source]Exponential functions in Statistical Thermodynamics play an important role. Most of the functions that are used in this area follow a gaussian distribution. Meaning, a normalized exponential function. Therefore, it is important the understanding of some of the basic properties of this type of functions.
In mathematics, exponential functions are in the form of:
Where is any constant, and appears as an exponent. This functions have the particularity to have a close relationship between its derivative and the function itself, due to direct relationship between both of them. However, probably the most relevant exponential function for the Statistical Thermodynamics has to be the exponential function with a base equal to Euler number (), which is the only constant of proportionality between its derivative and the function is 1.
On the other hand, exponential functions have logarithms as their inverse function, having the form of:
This function, also has a particular a relevant significance due to its mathematical properties. It is important to highlight that, along with the exponential function with base , logarithms with base are called natural logarithms.
Some of the properties of the exponential functions are:
Some of the properties of logarithm functions are:
Derivatives of Exponentials
[edit | edit source]Derivatives of Logarithms
[edit | edit source]Series
[edit | edit source]Notation
[edit | edit source]Key Relations
[edit | edit source]Derivatives of Series
[edit | edit source]See Also
[edit | edit source]
Tips for deriving relations with partition functions
Use the rules of logarithms
[edit | edit source]You can use the rules of logarithms to isolate terms.
Example
[edit | edit source]Derivatives of Constants are Equal to Zero
[edit | edit source]If you are taking a derivative of a function, you can use the rules of logarithms to isolate a term that depends on that variable. Now, you only need to take the derivative on this term. the derivatives of all other terms will be zero
Lagrange multipliers
Constrained Optimization
[edit | edit source]When constraints are imposed on a system, Lagrange multipliers can be incorporated to determine the maximum of the multivariable function. The new process for determining maximum of this function within the constraint becomes:
- Write the constraint as a function. i.e.,
- Define a new equation. where is an undefined constant
- Solve this set of equations to find the maximum, using the previous three steps for determining the maximum of an unconstrained system.
For example, suppose we have a function and we impose the following constraint upon the function:
The constraint would be written as
We would then define the new equation following the constraint as
Next, we take the partial derivative with respect for both x and y, set it to zero, and solve for x and y.
First of all taking the partial derivative with respect for x set to zero:
Evaluating this will give:
Now, the partial derivative with respect for y set to zero will be taken:
This leads to the following system of equations that can be solved to determine the maximum:
From Solving this set of equations the maximum subjected to the constraint of is found to be:
Unconstrained Optimization
[edit | edit source]Determining the maximum of an unconstrained system follows very similar steps it just will not have a lagrange multiplier as the system is not subjected to the
maximum along a given line, rather the maximum of the system itself. The steps to solve an unconstrained system becomes:
- Calculate the partial derivatives
- set them to zero
- solve for the variables
For example given the same function of first the partial derivatives will be calculated to be:
Let both partial derivatives be zero:
Finally the variables will be solved for. Giving a maximum that is different than that of the constrained system:
Translational partition function
Derivation of Translational Partition Function
[edit | edit source]A Molecular Energy State or is the sum of available translational, vibrational, rotational and electronic states available. The Translational Partition Function gives a "sum over" the available microstates.
The derivation begins with the fundamental partition function for a canonical ensemble that is classical and discrete which is defined as:
where j is the index, and is the total energy of the system in the microstate
For a particle in a 3D box with length , mass and quantum numbers the energy levels are given by:
Substituting the energy level equation for in the partition function
Using rules of summations we can split the above formula into a product of three summation formulas
Defining the dimensions of the box (Particle In A Box Model) in each direction to be equivalent
Because the spacings between translational energy levels are very small they can be treated as continuous and therefore approximate the sum over energy levels as an integral over n
Using the substitutions and The integral simplifies to
From The list of definite integrals the simplified integral has a known solution:
Therefore,
Re-substituting and
Since is length and
Rotational partition function of a linear molecule
Derivation
[edit | edit source]The rotational partition function, is a sum over state calculation of all rotational energy levels in a system, used to calculate the probability of a system occupying a particular energy level. The open form of the partition function is an infinite sum, as shown below. By making a few substitutions and replacing the sum with an integral, an algebraic expression for the rotational partition function can be derived.
The degeneracy, g, of a rotational energy level, j, is the number of different measurable states that have the same energy. For rotational energy levels, this is given by:
The rotational energy of a molecule is:
Substituting these values into the open form of the partition function, we get
Since the spacings of the rotational energy levels is small, the sum can be approximated as an integral over J,
From a table of integrals:
Letting x = J and we get
A symmetry factor is introduced to account for the nuclear spin states of homonuclear diatomic molecules. has a value of 2 for homonuclear diatomics and 1 for other linear molecules.
The rotational characteristic temperature is introduced to simplify the rotational partition function expression.
The physical meaning of the characteristic rotational temperature is an estimate of which thermal energy is comparable to energy level spacing. Substituting this into the partition function gives us
Vibrational partition function of a diatomic molecule
The general form of the molecular partition function is an infinite sum which is open form, making it difficult to calculate, this is why the sum is approximated as a closed form which leads to an algebraic equation. The derivation of the closed form on the equation is as follows:
The open form of the vibrational partition function:
solving for
(where j=0,1,2...)
and by substituting (i.e., singly degenerate) into the summation for the resulting equation is:
The j is taken outside the brackets by the common exponent rule:
Note that is the equation that represents the energy levels of a harmonic oscillator which is used to approximate the vibrational molecular degree of freedom. The vibrational zero point energy is not negligible and must be defined at n=0.
Next, in order for the open system to be converted into the closed system, the equation must take the form of a geometric series identity, like in calculus.
if x<1,
This is done by first letting
Then,
this converges when x<1, giving and by replacing x with the original expression, you have:
where is the vibrational frequency of the molecule, which can by found by the following equation: =
where k is the spring constant of the molecule and is the reduced mass
Example
[edit | edit source]Calculate the population of the ground vibrational state of at 298.15 K. ()
Next the Probability of the ground state can be calculated:
This means that 99.9998% of all molecules are in the ground vibrational state at 298.15 K.





































































































































































































































![{\displaystyle {\frac {\partial }{\partial a_{j}}}\left[\ln W(a_{1},a_{2},a_{3}...)-{\alpha }[({\Sigma _{i}a_{i}})-{\mathcal {A}}]-{\beta }[({\Sigma _{i}a_{i}E_{i}})-{\varepsilon }]\right]=0}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0a1559be18936c8de07ef799f3825672be21ff66)

![{\displaystyle -{\frac {\partial }{\partial a_{j}}}\ln {(a_{j}!)}=-{\frac {\partial }{\partial a_{j}}}[{a_{j}}\ln {(a_{j})}-{a_{j}}]={-a_{j}}{\frac {1}{a_{j}}}-\ln {(a_{j})}+{1}=-\ln {(a_{j})}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/99444426a8a4bba29897553281a3a46c77f45e9c)
![{\displaystyle {\frac {\partial }{\partial a_{j}}}\alpha [(\Sigma _{i}a_{i})-{\mathcal {A}}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/396eb480512feda6280936ae36fb0a2caab05a10)


![{\displaystyle {\frac {\partial }{\partial a_{j}}}{\beta }[({\Sigma _{i}a_{i}E_{i}})-{\varepsilon }]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/39f7b1280cd95c409b4df2ad530c90538320289f)


































































































































































































































![{\displaystyle K_{c}={\frac {[C]^{\nu _{C}}[D]^{\nu _{D}}}{[A]^{\nu _{A}}[B]^{\nu _{B}}}}={\frac {\rho _{C}^{\nu _{C}}\rho _{D}^{\nu _{D}}}{\rho _{A}^{\nu _{A}}\rho _{B}^{\nu _{B}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d9d476ca6f4480ddbe4e883c3bdfbfe4b63e43b1)



























![{\displaystyle \sum _{j}\nu _{j}[\ln(q_{j})-\ln(N_{j})]=0}](https://wikimedia.org/api/rest_v1/media/math/render/svg/30b43751f7232afe319151676a725cc71af0e2c3)

![{\displaystyle [\nu _{C}\ln(q_{C})-\nu _{C}\ln(N_{C})]+[\nu _{D}\ln(q_{D})-\nu _{D}\ln(N_{D})]-[\nu _{A}\ln(q_{A})-\nu _{A}\ln(N_{A})]-[\nu _{B}\ln(q_{B})-\nu _{B}\ln(N_{B})]=0}](https://wikimedia.org/api/rest_v1/media/math/render/svg/74937391fa845d7eebfbb1c5df4ee3f2c8adfbc3)

![{\displaystyle {\frac {N_{A}}{V}}={\frac {\#molecules}{volume}}=\rho _{A}=[A]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b2118ffccb33e9b7e1d347d83d2656a4509129ef)
































![{\displaystyle k[A]^{v_{a}}[B]^{v_{b}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/04444b893cbcb8218edf488f91e98ab204278f02)


![{\displaystyle {\ce {A+B<=>{}[C]^{\ddagger }}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6aae1d9631c9c68b5f13f1e359d1bf9475014d1d)
![{\displaystyle K^{\ddagger }={\frac {\ce {[C]^{\ddagger }}}{\ce {[A][B]}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cb54ef9dc837eff8cc17cec2f0c692d1bc13b4ae)




































































![{\displaystyle \left[\ln \left(p\right)\right]_{p_{0}}^{p}=-{\frac {A_{0}}{V}}{\left({\frac {k_{B}T}{2\pi m}}\right)}^{1/2}\left[t\right]_{0}^{t}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/aabcc51e97245026273e2fc42d56d5aa6419920e)


![{\displaystyle p=p_{0}\exp \left[-{\frac {A_{0}}{V}}\left({\frac {k_{B}T}{2\pi m}}\right)^{1/2}t\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/062769e1d070259190f54c0f227868bad826744f)






























































































![{\displaystyle K_{c}(T)={((q_{C}/V)^{\nu _{C}}(q_{D}/V)^{\nu _{D}}) \over ((q_{A}/V)]^{\nu _{A}}(q_{B}/V)^{\nu _{B}})}={{\rho _{C}}^{\nu _{C}}{\rho _{D}}^{\nu _{D}} \over {\rho _{A}}^{\nu _{A}}{\rho _{B}}^{\nu _{B}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/826fc96fd2865b4ba939fb9ae0e60f9ce5b19ac6)
























![{\displaystyle \log _{b}{\sqrt[{p}]{x}}={\frac {\log _{b}x}{p}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/85de3061851d6fb9347dc78ffcaed1775391138e)











![{\displaystyle {\frac {\partial }{\partial x}}[(-{2x}^{2}-{y}^{2}-{xy}-{10y})+{\alpha }({x}+{y}-{5})]=0}](https://wikimedia.org/api/rest_v1/media/math/render/svg/155ababf940378f9138de63ced50935ba0c8b857)


![{\displaystyle {\frac {\partial }{\partial y}}[(-{2x}^{2}-{y}^{2}-{xy}-{10y})+{\alpha }({x}+{y}-{5})]=0}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7976c5d821321bbb054db5c020f96613eb64eca9)















![{\displaystyle q_{trans}=\sum _{j}^{\infty }e^{-\beta [{\frac {h^{2}}{8mL^{2}}}(n_{x}^{2}+n_{y}^{2}+n_{z}^{2})]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0612537f2c630cf506517c4c435535f9227d97e8)
![{\displaystyle q_{trans}=\sum _{n_{x},n_{y},n_{z}=1}^{\infty }e^{-\beta [{\frac {h^{2}}{8mL^{2}}}(n_{x}^{2})]}e^{-\beta [{\frac {h^{2}}{8mL^{2}}}(n_{y}^{2})]}e^{-\beta [{\frac {h^{2}}{8mL^{2}}}(n_{z}^{2})]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6217fc6acd2bd9b15a6a8f53a3433ca081f3a7ab)


![{\displaystyle q_{trans}={\Bigg [}\sum _{n=1}^{\infty }e^{-\beta {\frac {h^{2}}{8mL^{2}}}(n^{2})}{\Bigg ]}^{3}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/dd4c45c26b41c3a61178d47b036d3e370e5d3d1c)









































![{\displaystyle \exp \left({\frac {-h\nu j}{k_{B}T}}\right)=\sum _{j=0}^{\infty }[\exp \left({\frac {-h\nu }{k_{B}T}}\right)]^{j}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/27931a1c8e1b97b9caa44ee13994dec9172d41fe)

















