Sensory Systems/Neurosensory Implants
Retinal Implants
[edit | edit source]Since the late 20th century, restoring vision to blind people by means of artificial eye prostheses has been the goal of numerous research groups and some private companies around the world. Similar to cochlear implants, the key concept is to stimulate the visual nervous system with electric pulses, bypassing the damaged or degenerated photoreceptors on the human retina. In this chapter we will describe the basic functionality of a retinal implant, as well as the different approaches that are currently being investigated and developed. The two most common approaches to retinal implants are called “epiretinal” and “subretinal” implants, corresponding to eye prostheses located either on top or behind the retina respectively. We will not cover any non-retina related approaches to restoring vision, such as the BrainPort Vision System that aims at stimulating the tongue from visual input, cuff electrodes around the optic nerve, or stimulation implants in the primary visual cortex.
Retinal Structure and Functionality
[edit | edit source]Figure 1 depicts the schematic nervous structure of the human retina. We can differentiate between three layers of cells. The first, located furthest away from the eye lens, consists of the photoreceptors (rods and cones) whose purpose is to transduce incoming light into electrical signals that are then further propagated to the intermediate layer, which is mainly composed of bipolar cells. These bipolar cells, which are connected to photoreceptors as well as cell types such as horizontal cells and amacrine cells, pass on the electrical signal to the retinal ganglion cells (RGC). For a detailed description on the functionality of bipolar cells, specifically with respect to their subdivision into ON- and OFF-bipolar cells, refer to chapter on Visual Systems. The uppermost layer, consisting of RGCs, collects the electric pulses from the horizontal cells and passes them on to the thalamus via the optic nerve. From there, signals are propagated to the primary visual cortex. There are some key aspects worth mentioning about the signal processing within the human retina. First, while bipolar cells, as well as horizontal and amacrine, generate graded potentials, the RGCs generate action potentials instead. Further, the density of each cell type is not uniform across the retina. While there is an extremely high density of rods and cones in the area of the fovea, with in addition only very few photoreceptors connected to RGCs via the intermediate layer, a far lower density of photoreceptors is found in the peripheral areas of the retina with many photoreceptors connected to a single RGC. The latter also has direct implications on the receptive field of a RGC, as it tends to increase rapidly towards the outer regions of the retina, simply because of the lower photoreceptor density and the increased number of photoreceptors being connected to the same RGC.
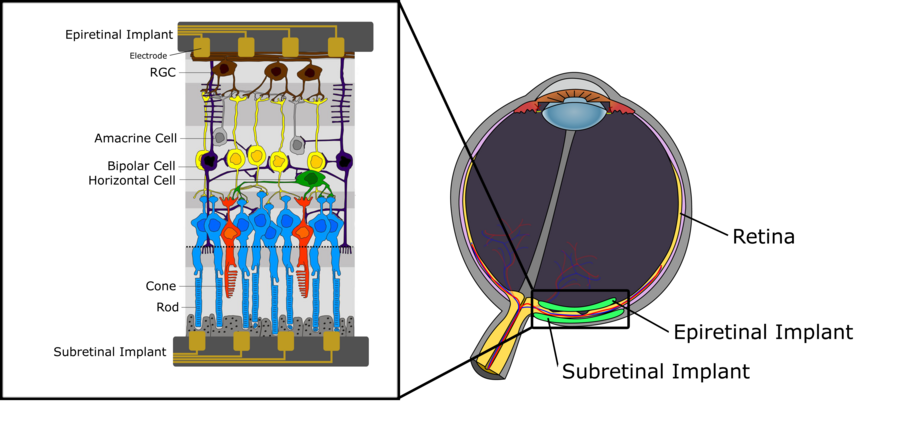
Implant Use Case: Retinal Degenerative Diseases
[edit | edit source]As mentioned previously in this wiki, the retina is a light-sensitive tissue located in the back of the eye consisting of different layers which contain a variety of cell types. The retina is primarily involved in neural visual processing with signals originating at photoreceptors and travelling to the brain by the axons of the ganglion cells. When this stratified tissue degenerates, permanent vision loss can occur [1]. This is often caused by retinal degenerative diseases such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP), which are the two most prevalent conditions that progressively lead to permanent visual impairments and loss. Currently, there are no cures for these two retinal diseases and with modern therapies only having the capacity to slow down disease progression, strategies are needed to restore patients’ vision. One of the tools currently being investigated is retinal prosthesis technology that stimulates viable retina tissue to reinstate vision, which will be described in a later section[2].
Age-Related Macular Degeneration (AMD)
[edit | edit source]As suggested by its name, macular degeneration is a retinal degenerative disease with an onset occurring primarily in elderly individuals. AMD revolves around the progressive degeneration of cone photoreceptors in the macula, leading to blurred vision in the center of the visual field. This can progress to a point where the individual has complete vision loss in the center of the visual field, known as blind spots. Though AMD can affect one or both eyes, it rarely leads to complete blindness, since the peripheral vision of the patient remains intact. There are two main types of AMD: dry and wet. Dry AMD accounts for the majority of the cases of the disease and is characterized by small yellow deposits, known as drusen, occurring in the macula between the retinal pigment epithelium and choroid. The progression in this form of AMD is initially slow with very few symptoms and only intensifies when retinal atrophy occurs. The wet form of AMD is characterized by choroid neovascularization, which is the abnormal growth of blood vessels that are prone to breaking and lead to blood, protein leakage, and scarring ultimately leading to permanent damage of the cones and therefore, vision loss. The progression of the wet form and vision loss is much more rapid than in dry AMD [3].
Retinitis Pigmentosa (RP)
[edit | edit source]

Retinitis Pigmentosa is an inherited degenerative eye disease involving rod photoreceptor cells that has an early onset in younger individuals. In this disease, the rods deteriorate progressively and eventually lead to vision loss in the periphery vision field as well as night vision. This loss first occurs externally then progresses inwards, creating an effect of “tunnel vision” in the patient. Visual impairment occurs symmetrically, with both eyes affected in similar timeframes. Unlike AMD, this eye disease can extend beyond the periphery and begin to affect the central visual field through degeneration of cone photoreceptor cells. This leaves the individual with continuous vision loss that can eventually lead to complete blindness, though quite rare. Retinitis Pigmentosa is genetically inherited and has a variety of gene mutations that can lead to an RP phenotype, leading to a variety of inheritance patterns. However, when the inheritance pattern is autosomal dominant, the majority of cases are linked to mutations in the rhodopsin gene. This mutation disrupts the function of rod-opsin, which is an essential protein in the phototransduction cascade. There is currently no cure for Retinitis Pigmentosa [2]. However, in 2008 Shigeru Sato and his colleagues discovered an extracellular matrix-like retinal protein named Pikachurin, which could lead to a potential disease therapy due to its involvement with interactions between photoreceptor cells and bipolar cells [4].
Microelectrode Arrays for Retina Stimulation
[edit | edit source]
As mentioned above, there are no cures for the progressive visual impairments caused by macular degeneration and Retinitis Pigmentosa. However, in both diseases, even though there is substantial photoreceptor cell loss, a significant amount of the inner retinal neurons survive years after disease onset. This provides an opportunity for artificial stimulation of the remaining, still properly functioning retina cells, through electrodes, to restore visual information for the human patient. Microelectrode arrays use electrodes to stimulate the retina extracellularly by tight placement that allows an electrochemical interface to be formed with the array and saline found around the retina. Current is injected through to the array-retina interface and ultimately drives the depolarization of the membranes of the neurons leading to action potentials. This stimulation can be cathodic or anodic. In cathodic stimulation, negative charges arise outside the membrane thereby driving positive charges intracellularly, resulting in a depolarization gradient that is strongest at close proximity to the electrode. In anodic stimulation, hyperpolarization occurs in the areas closest to the electrodes and depolarization occurs at further distances. Therefore, cathodic is generally viewed as more efficient for stimulation since it requires a much lower current injection. The phase of stimulation is not the only factor that affects the efficacy of stimulation. The waveform, which can take on a variety of shapes such as monophasic and biphasic, plays a large role in the safety of stimulation of retinal neurons. For example, in monkeys, it was found that a monophasic current with only an anodic phase could damage previously viable cells. Therefore, implants that use retinal stimulation will make use of a charge-balanced biphasic waveform. This waveform utilizes a cathodic phase for stimulation and an anodic phase for discharging, thereby balancing the charges around on the membrane. With this ability to stimulate, a retinal prosthetic can be implanted either behind the retina, and is then referred to as subretinal implant. This brings the electrodes closest to the damaged photoreceptors and the still properly functioning bipolar cells, which are the real stimulation target here. If the stimulation electrodes penetrate the choroid, which contains the blood supply of the retina, the implants are sometimes called "suprachoroidal" implants. Or the implant may be put on top of the retina, closest to the Ganglion cell layer, aiming at stimulation of the RGCs instead. These implants are referred to as epiretinal implants. Both approaches are currently being investigated by several research groups. They both have significant advantages as well as drawbacks. Before we treat them in more detail separately, we describe some key challenges that need consideration in both cases [2].
Challenges
[edit | edit source]Electrode Technology Challenges
[edit | edit source]A big challenge for retinal implants comes from the extremely high spatial density of nervous cells in the human retina. There are roughly 125 million photoreceptors (rods and cones) and 1.5 million ganglion cells in the human retina, as opposed to approximately only 15000 hair cells in the human cochlea [5] [6]. In the fovea, where the highest visual acuity is achieved, as many as 150000 cones are located within one square millimeter. While there are much fewer RGCs in total compared to photoreceptors, their density in the foveal area is close to the density of cones , imposing a tremendous challenge in addressing the nervous cells in high enough spatial resolution with artificial electrodes. Virtually all current scientific experiments with retinal implants use micro-electrode arrays (MEAs) to stimulate the retina cells. High resolution MEAs achieve an inter-electrode spacing of roughly 50 micrometers, resulting in an electrode density of 400 electrodes per square millimeter. Therefore, a one to one association between electrodes and photoreceptors or RGCs respectively is impossible in the foveal area with conventional electrode technology. However, spatial density of both photoreceptors as well as RGCs decrease s quickly towards the outer regions of the retina, making one-to-one stimulation between electrodes and peripheral nerve cells more feasible [7]. Another challenge is operating the electrodes within safe limits. Imposing charge densities above 0.1 mC/cm² may damage the nervous tissue [7]. Generally, the further a cell is away from the stimulating electrode, the larger is the current amplitude required for stimulation of the cell. Furthermore, the lower the stimulation threshold, the smaller the electrode may be designed and the compacter the electrodes may be placed on the MEAs, thereby enhancing the spatial stimulation resolution. Stimulation threshold is defined as the minimal stimulation strength necessary to trigger a nervous response in at least 50% of the stimulation pulses. For these reasons, a primary goal in designing retinal implants is to use as low a stimulation current as possible while still guaranteeing a reliable stimulation (i.e. generation of an action potential in the case of RGCs) of the target cell. This can either be achieved by placing the electrode as close as possible to the area of the target cell that reacts most sensitive to an applied electric field pulse or by making the cell projections, i.e. dendrites and/or axons, grow on top the electrode, allowing a stimulation of the cell with very low currents even if the cell body is located far away. Further, an implant fixed to the retina automatically follows the movements of the eyeball. While this entails some significant benefits, it also means that any connection to the implant – for adjusting parameters, reading out data, or providing external power for the stimulation – requires a cable that moves with the implant. As we move our eyes approximately three times a second, this exposes the cable and involved connections to severe mechanical stress. For a device that should remain functioning for an entire life time without external intervention, this imposes a severe challenge on the materials and technologies involved.
Biocompatibility Challenges
[edit | edit source]Besides electrical challenges, a key challenge in a retinal implant is its contact with biological tissue. When a foreign substance, such as an implant, comes into contact with physiological substances, an immune response is triggered. This response is typically in the form of inflammation or isolation of the substance, which often leads to scarring of the involved tissues. This is an issue especially with retinal implants because the prosthetic has to be inserted, through tissue, to the appropriate location. If the material used is too sharp or is not placed carefully, injury to the tissue can occur further intensifying an immune response. Additionally, these responses can lead to a loss of electrical signal over time as the immune system can “encapsulate” the stimulated area over time, making it difficult for a long-lasting implant. So far, one epi-retinal implant, Argus II, has been able to circumvent biocompatibility issues by having a retinal implant still functioning after 3 years in a patient. This implant makes use of silicone, which is a material that has good long term biocompatibility, but is a stiff substrate that doesn’t allow the device’s configuration to be easily modified. Other materials such as Polyimide and gold have been investigated for retinal implant functionality and biocompatibility. Polyimide is a promising polymer for future implants, since implants made of this material have been functional on human eyes in short-term studies. Such a material is advantageous due to its high biocompatibility, flexibility, and low costs. Optimization of materials suitable for retinal implants is ongoing as technological advances produce more complex microelectrode arrays that need different substrates for maximum functionality [8] [9].
Subretinal Implants
[edit | edit source]As the name already suggest, subretinal implants are visual prosthesis located behind the retina. Therefore, the implant is located closest to the damaged photoreceptors, aiming at bypassing the rods and cones and stimulating the bipolar cells in the next nervous layer in the retina. The main advantage of this approach lies in relatively little visual signal processing that takes place between the photoreceptors and the bipolar cells that need to be imitated by the implant. That is, raw visual information, for example captured by a video camera, may be forwarded directly, or with only relatively rudimentary signal processing respectively, to the MEA stimulating the bipolar cells, rendering the procedure rather simple from a signal processing point of view. However, this approach has some severe disadvantages. The high spatial resolution of photoreceptors in the human retina imposes a big challenge in developing and designing a MEA with sufficiently high stimulation resolution and therefore low inter-electrode spacing. Furthermore, the stacking of the nervous layers in z-direction (with the x-y plane tangential to the retina curvature) adds another difficulty when it comes to placing the electrodes close to the bipolar cells. With the MAE located behind the retina, there is a significant spatial gap between the electrodes and the target cells that needs to be overcome. As mentioned above, an increased electrode to target cell distance forces the MAE to operate with higher currents, enlarging the electrode size, the number of cells within the stimulation range of a single electrode and the spatial separation between adjacent electrodes. All of this results in a decreased stimulation resolution as well as opposing the retina to the risk of tissue damage caused by too high charge densities. As shown below, one way to overcome large distances between electrodes and the target cells is to make the cells grow their projections over longer distances directly on top the electrode.
In late 2010, a German research group in collaboration with the private German company “Retina Implant AG”, published results from studies involving tests with subretinal implants in human subjects [10] . A three by three millimeter micro-photodiode array (MPDA) containing 1500 pixels, which each pixel consisting of an individual light-sensing photodiodes and an electrode, was implanted behind the retina of three patients suffering from blindness due to macular degeneration. The pixels were located approximately 70 micrometer apart from each other, yielding a spatial resolution of roughly 160 electrodes per square millimeter – or, as indicated by the authors of the paper, a visual cone angle of 15 arcmin for each electrode. It should be noted, that, in contrast to implants using external video cameras to generate visual input, each pixel of the MPDA itself contains a light-sensitive photodiode, autonomously generating the electric current from the light received through the eyeball for its own associated electrode. So each MPDA pixel corresponds in its full functionality to a photoreceptor cell. This has a major advantage: Since the MPDA is fixed behind the human retina, it automatically drags along when the eyeball is being moved. And since the MPDA itself receives the visual input to generate the electric currents for the stimulation electrodes, movements of the head or the eyeball are handled naturally and need no artificial processing. In one of the patients, the MPDA was placed directly beneath the macula, leading to superior results in experimental tests as opposed to the other two patients, whose MPDA was implanted further away from the center of the retina. The results achieved by the patient with the implant behind the macula were quite extraordinary. He was able to recognize letters (5-8cm large) and read words as well as distinguish black-white patterns with different orientations [10].
The experimental results with the MPDA implants have also drawn attention to another visual phenomenon, revealing an additional advantage of the MPDA approach over implants using external imaging devices: Subsequent stimulation of retinal cells quickly leads to decreased responses, suggesting that retinal neurons become inhibited after being stimulated repeatedly within a short period of time. This entails that a visual input projected onto a MEA fixed on or behind the retina will result in a sensed image that quickly fades away, even though the electric stimulation of the electrodes remains constant. This is due to the fixed electrodes on the retina stimulating the same cells on the retina all the time, rendering the cells less and less sensitive to a constant stimulus over time. However, the process is reversible, and the cells regain their initial sensitivity once the stimulus is absent again. So, how does an intact visionary system handle this effect? Why are healthy humans able to fix an object over time without it fading out? As mentioned in [11], the human eye actually continuously adjusts in small, unnoticeable eye movements, resulting in the same visual stimulus to be projected onto slightly different retinal spots over time, even as we tend to focus and fix the eye on some target object. This successfully circumvents the fading cell response phenomenon. With the implant serving both as photoreceptor and electrode stimulator, as it is the case with the MPDA, the natural small eye adjustments can be readily used to handle this effect in a straight forward way. Other implant approaches using external visual input (i.e. from video cameras) will suffer from their projected images fading away if stimulated continuously. Fast, artificial jittering of the camera images may not solve the problem as this external movement may not be in accordance with the eye movement and therefore, the visual cortex may interpret this simply as a wiggly or blurry scene instead of the desired steady long term projection of the fixed image. A further advantage of subretinal implants is the precise correlation between stimulated areas on the retina and perceived location of the stimulus in the visual field of the human subject. In contrast to RGCs, whose location on the retina may not directly correspond to the location of their individual receptive fields, the stimulation of a bipolar cell is perceived exactly at that point in the visual field that corresponds to the geometric location on the retina where that bipolar cell resides. A clear disadvantage of subretinal implants is the invasive surgical procedure involved.
Epiretinal Implants
[edit | edit source]Epiretinal implants are located on top of the retina and therefore closest to the retina ganglion cells (RGCs). For that reason, epiretinal implants aim at stimulating the RGCs directly, bypassing not only the damaged photoreceptors, but also any intermediate neural visual processing by the bipolar, horizontal and amacrine cells. This has some advantages: First of all, the surgical procedure for an epiretinal implant is far less critical than for a subretinal implant, since the prosthesis need not be implanted from behind the eye. Also, there are much fewer RGCs than photoreceptors or bipolar cells, allowing a more course grained stimulation with increased inter-electrode distance (at least in the peripheral regions of the retina), or an electrode density even superior to that of the actual RGC density, allowing for more flexibility and accuracy when stimulating the cells. A study on the epiretinal stimulation of peripheral parasol cells conducted on macaque retina provides quantitative details [7]. Parasol cells are one type of RGCs forming the secondmost dense visual pathway in the retina. Their main purpose is to encode the movement of objects in the visual field, thus sensing motion. The experiments were performed in vitro by placing the macaque retina tissue on a 61 electrode MEA (60 micrometer inter-electrode spacing). 25 individual parasol cells were identified and stimulated electronically while properties such as stimulation threshold and best stimulation location were analyzed. The threshold current was defined as the lowest current that triggered a spike on the target cell in 50% of the stimulus pulses (pulse duration: 50 milliseconds) and was determined by incrementally increasing the stimulation strength until sufficient spiking response was registered. Please note two aspects: First, parasol cells as RGCs exhibit action potential behavior, as opposed to bipolar cells which work with graded potentials. Second, the electrodes on the MAE were both used for the stimulation pulses as well as for recording the spiking response from the target cells. 25 parasol cells were located on the 61 electrode MAE with a electrode density significantly higher than the parasol cell density, effectively yielding multiple electrodes within the receptive fields of a single parasol cell. In addition to measuring the stimulation thresholds necessary to trigger a reliable cell response, also the location of best stimulation was determined. The location of best stimulation refers to the location of the stimulating electrode with respect to the target cell where the lowest stimulation threshold was achieved. Surprisingly, this was found out to not be on the cell soma, as one would expect, but roughly 13 micrometers further down the axon path. From there on, the experiments showed the expected quadratic increase in stimulation threshold currents with respect to increasing electrode to soma distance. The study results also showed that all stimulation thresholds were well below the safety limits (around 0.05mC/cm², as opposed to 0.1mC/cm² being a (low) safety limit) and that the cell response to a stimulation pulse was fast (0.2 ms latency on average) and precise (small variance on latency). Further, the superior electrode density over parasol cell density allowed a reliable addressing of individual cells by the stimulation of the appropriate electrode, while preventing neighboring cells from also evoking a spike.
Overview of Alternative Technical Approaches
[edit | edit source]In this section, we give a short overview over some alternative approaches and technologies currently being under research.
Nanotube Electrode
[edit | edit source]Classic MAEs contain electrodes made out of titanium nitride or indium tin oxide exposing the implant to severe issues with long-term biocompatibility [12]. A promising alternative to metallic electrodes consists of carbon nanotubes (CNT) which combine a number of very advantageous properties. First, they are fully bio compatible since they are made from pure carbon. Second, their robustness makes them suited for long term implantation, a key property for visual prosthesis. Further, the good electric conductivity allows them to operate as electrodes. And finally, their very porous nature leads to extremely large contact surfaces, encouraging the neurons to grow on top the CNTs, thus improving the neuron to electrode contact and lowering the stimulation currents necessary to elicit a cell response. However, CNT electrodes have only emerged recently and at this point only few scientific results are available.
Wireless Implant Approaches
[edit | edit source]One of the main technical challenges with retinal implant relates to the cabling that connects the MEA with the external stimuli, the power supply as well as the control signals. The mechanical stress on the cabling affects its long term stability and durability, imposing a big challenge on the materials used. Wireless technologies could be a way to circumvent any cabling between the actual retinal implant and external devices. The energy of the incoming light through the eye is not sufficient to trigger neural responses. Therefore, to make a wireless implant work, extra power must be provided to the implant. An approach presented by the Stanford School of Medecine uses an infrared LCD display to project the scene captured by a video camera onto goggles, reflecting infrared pulses onto the chip located on the retina. The chip also uses a photovoltaic rechargeable battery to provide the power required to transfer the IR light into sufficiently strong stimulation pulses. Similar to the subretinal approach, this also allows the eye to naturally fix and focus onto objects in the scene, as the eye is free to move, allowing different parts of the IR image on the goggles to be projected onto different areas on the chip located on the retina. Instead of using infrared light, inductive coils can also be used to transmit electrical power and data signals from external devices to the implant on the retina. This technology has been successfully implemented and tested in the EPIRET3 retinal implant [13]. However, those tests were more a proof-of-concept, as only the patient’s ability to sense a visual signal upon applying a stimulus on the electrodes was tested.
Directed Neural Growth
[edit | edit source]One way to allow a very precise neural stimulation with extremely low currents and even over longer distances is to make the neurons grow their projections onto the electrode. By applying the right chemical solution onto the retinal tissue, neural growth can be encouraged. This can be achieved by applying a layer of Laminin onto the MEA’s surface. In order to control the neural paths, the Laminin is not applied uniformly across the MEA surface, but in narrow paths forming a pattern corresponding to the connections, the neurons should form. This process of applying the Laminin in a precise, patterend way, is called “microcontact printing”. A picture of what these Lamini paths look like is shown in Figure 5. The successful directed neural growth achieved with this method allowed applying significantly lower stimulation currents compared to classic electrode stimulation while still able to reliably trigger neural response [14]. Furthermore, the stimulation threshold no longer follows the quadratic increase with respect to electrode-soma distance, but remains constant at the same low level even for longer distances (>200 micrometer).
Microelectrode Arrays for Characterization of Retinal Function: A CMOS Based Technology
[edit | edit source]As explained earlier in the challenges section of retinal implants, many microelectrode arrays suffer from a large pitch and low number of electrodes, affecting their specificity and targeting of neurons in neural networks. This is a limiting factor in being able to see network dynamics and functionalities of neural populations. Specifically, many cellular details such as axonal propagation velocities and axonal information processing are lost in lower density arrays. Recently, researchers have taken advantage of complimentary-oxide-semiconductor (CMOS) technology to create high density microelectrode arrays with high spatial resolution that allow the detection of this cellular information as well as a high signal-to-noise ratio through platinum black deposition. Such arrays can have 26400 microelectrodes over sensing array of 3.85 x 2.10 mm². With a pitch of 17.5 μm, the electrode density is 3265 electrodes per μm² to accompany the 1024 readout channels [15] . With many switches below the electrodes, various electrode configurations can be used to assess the neural population on the chip. With such a sensitive and dense microelectrode chip, single cell identification, network level analysis, and axonal information can be recorded from neural cells. This technology opens the door to electrophysiological phenotypes “biomarkers” to be determined for disease modeling and for functionality of tissues since a dissected retina can be plated and recorded on a microelectrode array [16].
Retinal Recordings
[edit | edit source]Light signals are interpreted in the retina and this information is stored in the neurons of the ganglion layer, known as retinal ganglion cells (RGCs). These cells then send this information via action potentials which can be recorded by microelectrode arrays to understand retinal circuitry, development, and the encoding of a visual scene. These in vitro experiments are typically performed by first isolating the retina from its native tissue, plating the tissue with the retinal ganglion cells facing downwards on the array, and recording using light stimulation. Afterwards the data is analyzed using spike sorting, which will be explained later. Drug blockers and different light stimuli can be used to determine photoreceptor response and evaluate functionality. Furthermore, researchers can evaluate the effect of retinal mutations on RGC spiking behaviour to determine electrophysiological biomarkers. In one experiment, researchers used a microelectrode array for wild type mouse retinas and mice with a FRMD7 knockout. FMRD7 is a mutation associated with a horizontal, gaze-dependent rapid eye movements in affected individuals. The data from the recording sessions on the microelectrode array indicated that there was a loss of response to horizontal direction selective cells in the retina. The wild type mice did not have loss of response in either horizontal or vertical direction selective cells. Such a finding indicates the ability to use microelectrode array technology to determine electrophysiological biomarkers of retinal diseases in future research [17].

Spike Sorting
[edit | edit source]With the latest microelectrode technologies that allow neural recordings from thousands of electrodes, large quantities of simultaneous electrophysiological data from neural tissue and networks can be analyzed to unveil pertinent electrical information about the nervous system. When using a microelectrode array for neuroscience, electrical signals from neurons (action potentials) are recorded extracellularly. This means that the signal acquired in these recordings is the opposite of patch clamp; the amplitude of the action potential is negative as opposed to patch clamp. These extracellular signatures contain information not only about the action potentials, but also synaptic mechanisms (local field potentials), which can be identified through filtering and analysis. The process to analyze and assign this electrophysiological information to a single neuron is known as spike sorting.


The main aspect of a recording that is analyzed in a microelectrode recording is the spike-train. A neuron can be identified by its spiking activity since the timing of each event is dependent on the size, shape, and position of the neuron relative to the electrode. When recording from thousands of neurons, spike sorting becomes challenging to the cocktail party phenomenon. With multiple neurons in close vicinity to one another, it is very easy for an electrode to record signals from several neurons. Therefore, spike sorting has to identify a single neuron by its electrical “chatter” when there is a lot of background “chatter” occurring as well. Spike sorting is a multi-step process that takes the raw data from the neural population and assigns spikes to a single neuron despite this background noise.
The overview for the spike sorting process can have the following steps: Preprocessing raw data → Spike detection → Extraction of spikes and alignment → feature extraction → clustering → classification. In this general workflow, a spike sorting algorithm takes the raw data from the neural population and first preprocesses it by filtering out the low-frequency part of the action potential (noise). Spikes are then detected by setting a voltage threshold. Afterwards, the extracted spike waveforms need to be aligned with time in respect with a general feature of the action potential, such as its position. Then, the features are extracted from each individual waveform by using principal component analysis or wavelets, which is necessary for reducing the data to the necessary dimensions containing the information of interest. The spikes are then clustered so to create a template for a single neuron. This is done for the individual neurons in the data. There is not a “one size fits all” spike sorting algorithm as multielectrode recordings can differ between different cell types, species, and the type of recording done. Therefore, algorithms have to be adjusted and optimized to produce results that can accurately represent the raw data. However, once the data is spike sorted, a heap of information can be acquired from the data such as interspike intervals, refractory periods, and the ability to plot data of individual neurons against one another to detect differences [18].
Other Visual Implants
[edit | edit source]In addition to the stimulation of the retina, also other elements of the visual system can be stimulated
Stimulation of the Optic Nerve
[edit | edit source]With cuff-electrodes, typically with only a few segments.
Advantages:
- Little trauma to the eye.
Challenges:
- Not very specific.
Cortical Implants
[edit | edit source]
Dr. Mohamad Sawan, Professor and Researcher at Polystim neurotechnologies Laboratory at the Ecole Polytechnique de Montreal, has been working on a visual prosthesis to be implanted into the human cortex. The basic principle of Dr. Sawan’s technology consists in stimulating the visual cortex by implanting a silicium microchip on a network of electrodes made of biocompatible materials and in which each electrode injects a stimulating electrical current in order to provoke a series of luminous points to appear (an array of pixels) in the field of vision of the sightless person. This system is composed of two distinct parts: the implant and an external controller. The implant lodged in the visual cortex wirelessly receives dedicated data and energy from the external controller. This implantable part contains all the circuits necessary to generate the electrical stimuli and to oversee the changing microelectrode/biological tissue interface. On the other hand, the battery-operated outer control comprises a micro-camera which captures the image as well as a processor and a command generator which process the imaging data to select and translate the captured images and to generate and manage the electrical stimulation process and oversee the implant. The external controller and the implant exchange data in both directions by a powerful transcutaneous radio frequency (RF) link. The implant is powered the same way. (Wikipedia [1])
Advantages:
- Much larger area for stimulation: 2° radius of the central retinal visual field correspond to 1 mm² on the retina, but to 2100 mm² in the visual cortex.
Challenges:
- Implantation is more invasive.
- Parts of the visual field lie in a sulcus and are very hard to reach.
- Stimulation can trigger seizures.
Cochlear Implants
[edit | edit source]
A cochlear implant (CI) is a surgically implanted electronic device that replaces the mechanical parts of the auditory system by directly stimulating the auditory nerve fibers through electrodes inside the cochlea. Candidates for cochlear implants are people with severe to profound sensorineural hearing loss in both ears and a functioning auditory nervous system. They are used by post-lingually deaf people to regain some comprehension of speech and other sounds as well as by pre-lingually deaf children to enable them to gain spoken language skills. (Diagnosis of hearing loss in newborns and infants is done using otoacoustic emissions, and/or the recording of auditory evoked potentials.) A quite recent evolution is the use of bilateral implants allowing recipients basic sound localization.
Parts of the cochlear implant
[edit | edit source]The implant is surgically placed under the skin behind the ear. The basic parts of the device include:
External:
- a microphone which picks up sound from the environment
- a speech processor which selectively filters sound to prioritize audible speech and sends the electrical sound signals through a thin cable to the transmitter,
- a transmitter, which is a coil held in position by a magnet placed behind the external ear, and transmits the processed sound signals to the internal device by electromagnetic induction,
Internal:

- a receiver and stimulator secured in bone beneath the skin, which converts the signals into electric impulses and sends them through an internal cable to electrodes,
- an array of up to 24 electrodes wound through the cochlea, which send the impulses to the nerves in the scala tympani and then directly to the brain through the auditory nerve system
Signal processing for cochlear implants
[edit | edit source]In normal hearing subjects, the primary information carrier for speech signals is the envelope, whereas for music, it is the fine structure. This is also relevant for tonal languages, like Mandarin, where the meaning of words depends on their intonation. It was also found that interaural time delays coded in the fine structure determine where a sound is heard from rather than interaural time delays coded in the envelope, although it is still the speech signal coded in the envelope that is perceived.
The speech processor in a cochlear implant transforms the microphone input signal into a parallel array of electrode signals destined for the cochlea. Algorithms for the optimal transfer function between these signals are still an active area of research. The first cochlear implants were single-channel devices. The raw sound was band-passed filtered to include only the frequency range of speech, then modulated onto a 16 kHz wave to allow the electrical signal to electrically couple to the nerves. This approach was able to provide very basic hearing, but was extremely limited in that it was completely unable to take advantage of the frequency-location map of the cochlea.
The advent of multi-channel implants opened the door to try a number of different speech-processing strategies to facilitate hearing. These can be roughly divided into Waveform and Feature-Extraction strategies.
Waveform Strategies
[edit | edit source]These generally involve applying a non-linear gain on the sound (as an input audio signal with a ~30dB dynamic range must be compressed into an electrical signal with just a ~5dB dynamic range), and passing it through parallel filter banks. The first waveform strategy to be tried was Compressed Analog approach. In this system, the raw audio is initially filtered with a gain-controlled amplifier (the gain-control reduces the dynamic range of the signal). The signal is then passed through parallel band-pass filters, and the output of these filters goes on to stimulate electrodes at their appropriate locations.
A problem with the Compressed Analog approach was that the there was a strong interaction-effect between adjacent electrodes. If electrodes driven by two filters happened to be stimulating at the same time, the superimposed stimulation could cause unwanted distortion in the signals coming from hair cells that were within range of both of these electrodes. The solution to this was the Continuous Interleaved Sampling Approach - in which the electrodes driven by adjacent filters stimulate at slightly different times. This eliminates the interference effect between nearby electrodes, but introduces the problem that, due to the interleaving, temporal resolution suffers.
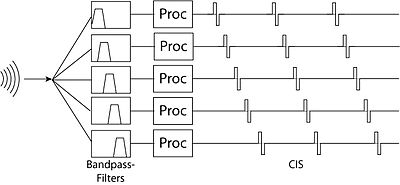
Feature-Extraction Strategies
[edit | edit source]These strategies focus less on transmitting filtered versions of the audio signal and more on extracting more abstract features of the signal and transmitting them to the electrodes. The first feature-extraction strategies looked for the formants (frequencies with maximum energy) in speech. In order to do this, they would apply wide band filters (e.g. 270 Hz low-pass for F0 - the base formant, 300 Hz-1 kHz for F1, and 1 kHz-4 kHz for F2), then calculate the formant frequency, using the zero-crossings of each of these filter outputs, and formant-amplitude by looking at the envelope of the signals from each filter. Only electrodes corresponding to these formant frequencies would be activated. The main limitation of this approach was that formants primarily identify vowels, and consonant information, which primarily resides in higher frequencies, was poorly transmitted. The MPEAK system later improved on this design my incorporating high-frequency filters which could better simulate unvoiced sounds (consonants) by stimulating high-frequency electrodes, and formant frequency electrodes at random intervals.[19][20][21]
Current Developments
[edit | edit source]
Currently, the leading strategy is the SPEAK system, which combines characteristics of Waveform and Feature-Detection strategies. In this system, the signal passes through a parallel array of 20 band-pass filters. The envelope is extracted from each of these and several of the most powerful frequencies are selected (how many depends on the shape of the spectrum), and the rest are discarded. This is known as a 'n-of-m" strategy. The amplitudes of these are then logarithmically compressed to adapt the mechanical signal range of sound to the much narrower electrical signal range of hair cells.
Multiple microphones
[edit | edit source]On its newest implants, the company Cochlear uses 3 microphones instead of one. The additional information is used for beam-forming, i.e. extracting more information from sound coming from straight ahead. This can improve the signal-to-noise ratio when talking to other people by up to 15dB, thereby significantly enhancing speech perception in noisy environments.
Integration CI – Hearing Aid
[edit | edit source]Preservation of low-frequency hearing after cochlear implantation is possible with careful surgical technique and with careful attention to electrode design. For patients with remaining low-frequency hearing, the company MedEl offers a combination of a cochlea implant for the higher frequencies, and classical hearing aid for the lower frequencies. This system, called EAS for electric-acoustic stimulation, uses with a lead of 18mm, compared to 31.5 mm for the full CI. (The length of the cochlea is about 36 mm.) This results in a significant improvement of music perception, and improved speech recognition for tonal languages.
Fine Structure
[edit | edit source]
For high frequencies, the human auditory system uses only tonotopic coding for information. For low frequencies, however, also temporal information is used: the auditory nerve fires synchronously with the phase of the signal. In contrast, the original CIs only used the power spectrum of the incoming signal. In its new models, MedEl incorporates the timing information for low frequencies, which it calls fine structure, in determining the timing of the stimulation pulses. This improves music perception, and speech perception for tonal languages like Mandarin.
Mathematically, envelope and fine-structure of a signal can be elegantly obtained with the Hilbert Transform (see Figure). The corresponding Python code is available under.[22]
Virtual Electrodes
[edit | edit source]The numbers of electrodes available is limited by the size of the electrode (and the resulting charge and current densities), and by the current spread along the endolymph. To increase the frequency specificity, one can stimulate two adjacent electrodes. Subjects report to perceive this as a single tone at a frequency intermediate to the two electrodes.
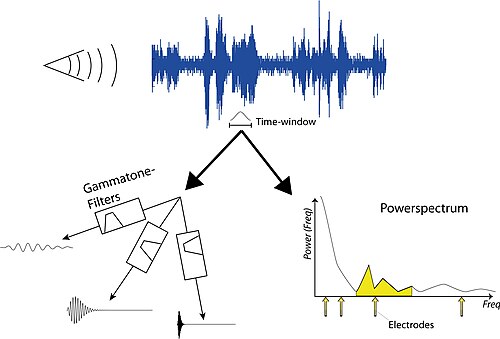
Simulation of a cochlear implant
[edit | edit source]Sound processing in cochlear implant is still subject to a lot of research and one of the major product differentiations between the manufacturers. However, the basic sound processing is rather simple and can be implemented to gain an impression of the quality of sound perceived by patients using a cochlear implant. The first step in the process is to sample some sound and analyze its frequency. Then a time-window is selected, during which we want to find the stimulation strengths of the CI electrodes. There are two ways to achieve that: i) through the use of linear filters ( see Gammatone filters); or ii) through the calculation of the powerspectrum (see Spectral Analysis).
Cochlear implants and Magnetic Resonance Imaging
[edit | edit source]With more than 150 000 implantations worldwide, Cochlear Implants (CIs) have now become a standard method for treating severe to profound hearing loss. Since the benefits of CIs become more evident, payers become more willing to support CIs and due to the screening programs of newborns in most industrialized nations, many patients get CIs in infancy and will likely continue to have them throughout their lives. Some of them may require diagnostic scanning during their lives which may be assisted by imaging studies with Magnetic resonance imaging (MRI). For large segments of the population, including patients suffering from stroke, back pain or headache, MRI has become a standard method for diagnosis. MRI uses pulses of magnetic fields to generate images and current MRI machines are working with 1.5 Tesla magnet fields. 0.2 to 4.0 Tesla devices are common and the radiofrequency power can peak as high as 6 kW in a 1.5 Tesla machine.
Cochlear implants have been historically thought to incompatible with MRI with magnetic fields higher than 0.2 T. The external parts of the device always have to be removed. There are different regulations for the internal parts of the device. Current US Food and Drug Administration (FDA) guidelines allow limited use of MRI after CI implantation. The pulsar and Sonata (MED-EL Corp, Innsbruck, Austria) devices are approved for 0.2 T MRI with the magnet in place. The Hi-res 90K (Advanced Bionics Corp, Sylmar, CA, USA) and the Nucleus Freedom (Cochlear Americas, Englewood, CO, USA) are approved for up to 1.5 T MRI after surgical removal of the internal magnet. Each removal and replacement of the magnet can be done using a small incision under local anesthesia, but the procedure is likely to weaken the pocket of the magnet and to risk infection of the patient.
Cadaver studies have shown that there is a risk that the implant may be displaced from the internal device in a 1.5 T MRI scanner. However, the risk could be eliminated when a compression dressing was applied. Nevertheless, the CI produces an artifact that could potentially reduce the diagnostic value of the scan. The size of the artifact will be larger relative to the size of the patient’s head and this might be particularly challenging for MRI scans with children. A recent study by Crane et al., 2010 found out that the artifact around the area of the CI had a mean anterior-posterior dimension of 6.6 +/- 1.5 cm (mean +/- standard deviation) and a left-right dimension averaging 4.8 +/- 1.0 cm (mean +/- standard deviation) (Crane et al., 2010). ([23])
Vestibular Implants
[edit | edit source]Introduction
[edit | edit source]People with damaged vestibular systems experience a combination of symptoms that may include hearing and vision disturbances, vertigo, dizziness, and spatial disorientation. Currently, there are no effective treatments for patients with weak or damaged vestibular systems. Over the past decade, scientists have developed an electrical stimulating device, similar to cochlear implants, that would restore semicircular canal function. Vestibular implants are intended to restore balance in patients with a damaged vestibular system. Figure[24] shows a vestibular implant prototype, which is a modified cochlear implant designed by MED-EL (Innsbruck, Austria).

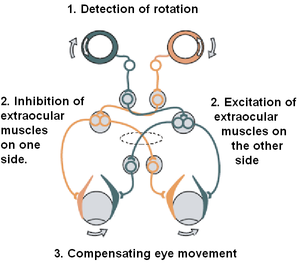
This vestibular neuroprosthesis prototype contains four major components: an electrical stimulator, three extracochlear electrodes that are placed in the ampullae of each semicircular canal, and an intracochlear array. When the vestibular implant is turned on, trains of electrical stimulation in the form of charge-balance, biphasic pulses are delivered down each extracochlear electrode toward a respective vestibular nerve [24]. Ultimately, the electrical stimulation would restore balance in a patient by stabilizing gaze via the vestibulo-ocular reflex (VOR). Progress toward an implantable prosthesis has shown promising results to effectively restore normal vestibular sensory transduction of head rotations. However, achieving an accurate stimulation paradigm to chronically encode three-dimensional head movements without causing undesired neuronal activity remains one of several key challenges.
Vestibular prosthesis evolution (1963-2014)
[edit | edit source]In 1963, Cohen and Suzuki [25] introduced the notion of vestibular prosthesis by demonstrating that eye movements can be induced via electrical stimulation of the ampullary branch of a vestibular nerve. Studies that followed were driven to engineer a continuous and accurate stimulation model for rehabilitating patients with different types of vestibular disorders, such as bilateral loss of vestibular function (BVL) and Meniere's disease [24] [26]. Four decades after Cohen and Sukui's pioneering work, Merfeld and colleagues developed the first vestibular device for generating smooth eye movements by electrically stimulating the vestibular nerve [27] [28]. The feasibility of neuro-electronic vestibular devices had further inspired researchers to integrate a motion-detection system to measure head movements. Santina and colleagues [29] [30] [31] [32] used gyroscopic sensors to measure movements in three-dimensional space and encoded this information to generate signals that control muscles of each eye via the vestibular nerve. As of late 2012, only two groups in the world have conducted vestibular implant studies on humans: a team led by Jay Rubinstein at the University of Washington and a joint-effort between a team led by Herman Kingma at the Maastrict University of Medical Center in the Netherlands and second group led by Jean-Phillippe Guyot at Hopitaux Universitaries de Geneve, Switzerland [24]. Jay Rubinstein led the first vestibular clinical study in 2010. Rubinstein and colleagues had successfully installed a vestibular pacemaker to reduce or cease involuntary vertigo attacks in patients diagnosed with Meniere's disease [26]. This device was combined with a handheld controller to start and stop a range of electrical stimuli that can be directed to any or all electrodes, but did not code for motion [26]. Unfortunately, the vestibular pacemaker in implanted patients had resulted in both the auditory and vestibular function deteriorating considerably [33] [26] [24]. A new direction has been taken from this group to explore a different electrical stimulation paradigm by incorporating information about motion [33]. The second attempt for human clinical studies was carried by Kingma, Guyot, and colleagues in 2012. Vestibular implants used in this study were prototyped by MED-EL. Perez-Fornos and colleagues [24] demonstrated that patients achieved a level of satisfactory functional recovery that allows them to exercise everyday activities such as walking.
Current progress is being made through ongoing university-industry partnerships. There are four leading University and/or industry partnerships working toward a vestibular prosthesis for clinical applications. These teams include: Rubinstein at the University of Washington and Cochlear Ltd (Lane Cove, Australia), Della Santina's team at the Vestibular NeuroEngineering Laboratory [Johns Hopkins School of Medicine, Baltimore, MD, USA], Daniel Merfeld's team at the Jenks Vestibular Physiology Laboratory at Harvard [Massachusetts Eye and Ear Infirmary, Boston, MA, USA], and a joint-effort between Herman Kingma, Jean-Philippe Guyot, and MED-EL.
Future directions in research
[edit | edit source]The state-of-the-art vestibular implant technology is a two-step system that produces electrical stimulations to three ampullary nerves in response to rotations around a respective axis (anterior, posterior, or horizontal canals). However, the biophysics of prosthetic nerve stimulation remains a challenge to mimic normal sensory transduction. Even though much is already known about how vestibular nerve afferents encode head movements, it is not yet understood how to design a noninvasive stimulus encoding strategy for a multichannel prosthesis. Active research has continued to focus on overcoming design and signal transduction limitations.
Current neural prostheses are intended to excite neural tissues in which they are implanted, but the effect of continuous excitatory stimulations can yet cause neurological deficits
[26].
Ultimately, a device that can both excite head motion in one direction and inhibit movement in the opposite direction is much desired. The latest prototype system developed by Santina and colleagues, SCSD1, has shown that direct current stimulations can evoke excitatory and inhibitory VOR responses
[34].
Their results demonstrate that effects of introducing the vestibular system to an artificial baseline can possibly alter the dynamic ranges of excitatory and inhibitory thresholds in unpredicted ways. On the other hand, clinical studies show that it is possible for humans to adapt within a reasonably short time (a few minutes) to the absence and presence of artificial neural activity
[35].
Once adaptation is reached, then one can tune the amplitude and frequency modulations of the stimulation to elicit smooth eye movements of different speeds and directions
[35].
Another type of design limitation of electrical prosthesis is current to spread away from the targeted nerve tissue and cause stimulations in the wrong canal
[36]
[37].
As a consequence, this current spread induces misalignment between the axis of the eye and head rotation
[38].
Therefore, the mechanisms underlying directional neural plasticity can provide well-aligned responses for humans. Other studies suggest infrared nerve stimulation is advantageous for targeting specific neurons and less obtrusive to nearby populations of neurons
[36]
[38].
The use of optics would allow higher spatial selectivity and improved surgical access
[36].
In addition, a fundamental challenge underlying the development of vestibular prosthesis is accounting for ways in which information from vestibular end organs can elicit particular movements. It has been shown that reflex and perceptual responses are dependent on which vestibular afferent inputs are stimulated
[33].
Surgical practices are examined for accurate placements of the electrode with respect to the afferents, which in the end could greatly influence the ability to stimulate a desired response.
Because the auditory and vestibular areas of the inner ear are connected, the spread of current beyond the target ampullary nerves and/or risks of surgery could interfere with cochlear nerve activity. It is likely that humans with implants will experience a risk of hearing loss, as observed in rhesus monkeys
[39].
Santina and colleagues
[39]
found that implantation of electrodes caused up to 14 dB of hearing loss and delivery of electrical stimulation further reduced hearing by 0.4-7.8 dB. This study suggests that current spread to cochlear hair cells may cause random activity in nearby cochlear regions.
Olfactory Implants
[edit | edit source]
Anosmia (Loss of Smell) appears in about 5% of the general population. An intact olfactory system is a core part of the perception of flavor with drinking and eating. Most problems presenting with taste loss come from an olfactory disorder. Additionally, the reception of smell is also central to our quality of life. Many experiences, such as a spring showers, fresh flowers or the scent of home add to any event, even if they are difficult to describe. While inflammatory causes of smell loss can be solved with the use of topical and systemic steroids, many treatments for other common causes of anosmia, including upper respiratory infection (URI), head trauma, and aging have not proven effective.

Feasibility study
[edit | edit source]A Study by Eric H. Holbrook, Sidharth V. Puram and others was done to determine the feasibility of inducing smell through artificial electrical stimulation of the olfactory bulbs in humans. Five subjects (age, 43–72 years) were enrolled. Three subjects reported a perception of smell with electrical stimulation. All subjects tolerated the study with minimal discomfort. The test subjects were all able to perceive smell, which was confirmed with a commercially available, 40-item, scratch-and-sniff identification test. Under endoscopic guidance and without topical anesthetic, a monopolar or bipolar electrode was positioned at 3 areas along the lateral lamella of the cribriform plate at the junction with the skull base: (1) the anterior ethmoid posterior to the frontal sinus opening; (2) the posterior ethmoid anterior to the sphenoid face; and (3) the middle ethmoid approximated by half the distance between the anterior and posterior points[40]. During 0.2-0.3ms, the implants were stimulated with an intensity range from 1 to 20 mA. 3 of the 5 subjects reported an experience of smell yet could not clearly state what that smell resembles and had differences among each other. The perception of smell did not change majorly with different intensities or electrode location, but small deviations described as “sweet,” “sour,” or “bad” were reported. There were no differences between monopolar or bipolar electrodes. The perceived smell was described as “onion-like,” “antiseptic like” or “sour,” and “fruity” or ”bad.” When asked to rank the perceived intensities of the smell on a scale from 1 to 10, the result ranged from 2 to 4. All subjects also experienced some discomfort with the devices, which presented as a throbbing, tingling, or pulsing sensation located at the ground electrode, the inner canthus of the eye or bridge of the nose, nasal tip, or in one case deep behind the eye. Electrodes that were positioned in the olfactory cleft caused sneezing or discomfort in four of the subjects and in the only one that tolerated it resulted in no perception of smell. In conclusion, the study achieved the perception of smell with electrical stimulation of the olfactory bulb for the first time. The authors plan to further explore the use of such implants, stating “Future work will extend the trials to include subjects without a sense of smell and develop more consistent objective measurements of olfactory perception.” This study was only intended as a proof of concept for future research into the possibility of restoring the olfaction from smell loss with electrical stimulation technology[41].
Future Directions
[edit | edit source]Electronic measurement of odors
[edit | edit source]Nowadays odors can be measured electronically in a huge amount of different ways, some examples are: mass spectrography, gas chromatography, raman spectra and most recently electronic noses. In general they assume that different olfactory receptors have different affinities to specific molecular physicochemical properties, and that the different activation of these receptors gives rise to a spatio-temporal pattern of activity that reflects odors.
Electronic Nose
[edit | edit source]
E-noses are artificial odor sensing devices based on a chemosensor array and pattern recognition. They are used to identify and quantify substances dissolved in air (or other carrier substances). An e-nose consists of a sampling device (analog to the nose), a sensor array (analog to the olfactory receptor neurons) and a computing unit (analog to the brain).
Sensor arrays
[edit | edit source]Like in the animal noses, unspecific sensors are used. This is not only due to the fact that it is very hard to find very specific sensors, but one also wants to cover a huge range of possible compounds without a sensor for each of them. Furthermore it is more robust, precise and efficient if the processing is based on information of more than one sensor. Such sensors experience a change in their electrical properties (E.g. higher resistance) when they come in contact with a compound. This alteration leads to a voltage change that is digitized (AD Converter).
The most frequently used sensor types include metal oxide semiconductors (MOS), quartz crystal microbalances (QCM), conducting polymers (CP) and surface acoustic wave (SAW) sensors. Another promising technology is bioelectronic noses that use proteins as sensors. It is also possible to use a combination of different sensors to get a more precise result and to combine the advantages of several sensor types, e.g better temporal responsivity versus better sensitivity.
Example: working principle of a conducting polymer sensor
[edit | edit source]A conducting polymer sensor consists of an array of about 2-40 different conducting polymers (long chains of organic molecules). Some odor molecules permeate into the polymer film and cause the film to expand thereby increasing its resistance. This increase in resistance of many polymer types can be explained by percolation theory.[42] Due to the chemical properties of the materials, different polymers react differently to the same odor.
Computation
[edit | edit source]The sensor signal has to be matched to an odorant mixture with a pattern recognition algorithm. It is possible to create a database of potential combinations and find the best match with multivariate statistical methods when an odor is presented or a neural network can be trained to recognize the patterns. Often also principal component analysis is used to reduce the dimensionality of the sensor data.
Applications
[edit | edit source]There are many applications for e-noses. They are used in aerospace and other industry to detect and monitor hazardous or harmful substances and for quality control. Possible applications in security are drug or explosive detection. E-noses may someday be able to replace police dogs. A very powerful application could be the diagnosis of diseases that alter the chemical composition of breath or the smell of excretions or blood, thereby potentially substituting invasive diagnostic techniques. It can also be employed to diagnose cancer, as certain cancer cells can be identified by their volatile organic compound profile. Cancer diagnosis by smell has already been found to work with dogs, flies,[43] but practically suitable methods with high sensitivity and specificity are still under development. Another medical application is the treatment of anosmia (inability to perceive odor) by an olfactory implant on basis of an e-nose. This too is still in development. In contrast, e-noses are already in use for environmental monitoring and protection. In robotics, e-noses could be used to follow airborne smells or smells on the ground. Especially for robotics it would be very interesting to have a better understanding of the insect’s olfactory system, since, in order to use the smell to navigate or to locate odor sources the often neglected temporal stimulus information has to be used.
Insects can follow odors as they can react to changes within about 150 milliseconds, and some of their receptors are able to depict fast odor concentration changes that occur in frequencies above at least 10 Hz. In contrast, conducting polymer as well as metal oxide e-noses have response times in the range of seconds to minutes [42] with only few exceptions reported in the range of tens of milliseconds.
References
[edit | edit source]- ↑ Larry Squire; et al. (2012). Fundamental Neuroscience 4th edition.
{{cite book}}: Explicit use of et al. in:|author=(help) - ↑ a b c Lan Yue, James D. Weiland, Botond Roska, Mark S. Humayun (2016). "Retinal stimulation strategies to restore vision: Fundamentals and Systems".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Jackson, G.R., Owsley, C., Curcio, C.A (2002). "Photoreceptor degeneration and dysfunction in aging and age-related maculopathy".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Shigeru Sato, Yoshihiro Omori; et al. (2008). "Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation".
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ↑ Jost B. Jonas, UlrikeSchneider, Gottfried O.H. Naumann (1992). "Count and density of human retinal photoreceptors". Springer.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Ashmore Jonathan (2008). "Cochlear Outer Hair Cell Motility". American Physiological Society.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ a b c Chris Sekirnjak, PawelHottowy, Alexander Sher, Wladyslaw Dabrowski, Alan M. Litke, E.J. Chichilnisky (2008). "High-Resolution Electrical Stimulation of Primate Retina for Epiretinal Implant Design". Society of Neuroscience.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Jong-Mo Seo; et al. (2004). "Biocompatibility of polyimide microelectrode array for retinal stimulation".
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ↑ Eui Tae Kim; et al. (2009). "Feasibility of Microelectrode Array (MEA) Based on Silicone-Polyimide hybrid for retina prosthesis".
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ↑ a b Eui Ta Eberhart Zrenner, KarlUlrich Bartz-Schmidt, Heval Benav, Dorothea Besch, Anna Bruckmann, Veit-Peter Gabel, Florian Gekeler, Udo Greppmaier, Alex Harscher, Steffen Kibbel, Johannes Koch, Akos Kusnyerik, tobias Peters, Katarina Stingl, Helmut Sachs et al.e Kim; et al. (2010). "Subretinal electronic chips allow blind patients to read letters and combine them to words".
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ↑ Pritchard Roy. "Stabilized Images on the Retina".
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Asaf Shoval, ChrisopherAdams, Moshe David-Pur, Mark Shein, Yael Hanein, Evelyne Sernagor (2009). "Carbon nanotube electrodes for effective interfacing with retinal tissue".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Susanne Klauke, Michael Goertz, Stefan Rein, Dirk Hoehl, Uwe Thomas, Reinhard Eckhorn, Frank Bremmer, Thomas Wachtler (2011). "Stimulation with a Wireless Intraocular Epiretinal Implant Elicits Visual Percepts in Blind Humans". The Association for Research in Vision and Ophthalmology.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Neville Z. Mehenti, GrehS. Tsien, Theodore Leng, Harvey A. Fishman, Stacey F. Bent (2006). "A model retinal interface based on directed neuronal growth for single cell stimulation". Springer.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Jan Muller; et al. (2015). "High-resolution CMOS MEA platform to study neurons at subcellular, cellular, and network levels".
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ↑ Fiscella M; et al. (2012). "Recording from defined populations of retinal ganglion cells using a high-density cmos-integrated microelectrode array with real-time switchable electrode selection".
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help) - ↑ Fiscella M, Yonehara K, Drinnenberg A, Franke F, Müller J, Roska B and Hierlemann A (2016). "Screening Transgenic Mouse Models of Human Eye Diseases with CMOS High-Density Microelectrode Arrays".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Gaute T Einevoll, Felix Franke, Espen Hagen, Christophe Pouzat, and Kenneth D Harris (2012). "Towards reliable spike-train recordings from thousands of neurons with multielectrodes".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ http://www.utdallas.edu/~loizou/cimplants/tutorial/tutorial.htm
- ↑ www.ohsu.edu/nod/documents/week3/Rubenstein.pdf
- ↑ www.acoustics.bseeber.de/implant/ieee_talk.pdf
- ↑ T. Haslwanter (2012). "Hilbert Transformation [Python]". private communications.
- ↑ Crane BT, Gottschalk B, Kraut M, Aygun N, Niparko JK (2010) Magnetic resonance imaging at 1.5 T after cochlear implantation. Otol Neurotol 31:1215-1220
- ↑ a b c d e f
Perez Fornos, A.; Guinand, N.; Van De Berg, R.; Stokroos, R.; Micera, S.; Kingma, H.; Pelizzone, M.; and Guyot, J. (2014). "Artificial balance: restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis". Frontiers in Neurology. 5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Cohen, B. and Suzuki, J. (1963). "Eye movements induced by ampullary nerve stimulation". The American journal of physiology. 204: 347–351.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ a b c d e
Golub, J. S.; Ling, L.; Nie, K.; Nowack, A.; Shepherd, S. J.; Bierer, S. M.; Jameyson, E.; Kaneko, C. R.; Phillips, J. O.; and Rubinstein, J. T. (2014). "Prosthetic Implantation of the Human Vestibular System". Otology & Neurotology. 1: 136–147.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Gong, W. and Merfeld, D. M. (2000). "Prototype neural semicircular canal prosthesis using patterned electrical stimulation". Annals of Biomedical Engineering. 28: 572–581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Lewis, R. F.; Haburcakova, C.; Gong, W.; Makary, C.; and Merfeld, D. M. (2010). "Vestibuloocular Reflex Adaptation Investigated With Chronic Motion-Modulated Electrical Stimulation of Semicircular Canal Afferents". Journal of Neurophysiology. 103: 1066–1079.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Dai, C.; Fridman, G. Y.; Chiang, B.; Davidovics, N.; Melvin, T.; Cullen, K. E. and Della Santina, Charles C. (2011). "Cross-axis adaptation improves 3D vestibulo-ocular reflex alignment during chronic stimulation via a head-mounted multichannel vestibular prosthesis". Experimental Brain Research. 210: 595–606.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Dai, C.; Fridman, G. Y.; Davidovics, N.; Chiang, B.; Ahn, J. and Della Santina, C. C. (2011). "Restoration of 3D Vestibular Sensation in Rhesus Monkeys Using a Multichannel Vestibular Prosthesis". Hearing Research. 281: 74–83.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Dai, Chenkai and Fridman, Gene Y. and Chiang, Bryce and Rahman, Mehdi A. and Ahn, Joong Ho and Davidovics, Natan S. and Della Santina, Charles C. (2013). "Directional Plasticity Rapidly Improves 3D Vestibulo-Ocular Reflex Alignment in Monkeys Using a Multichannel Vestibular Prosthesis". Journal of the Association for Research in Otolaryngology. 14: 863–877.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Davidovics, Natan S. and Rahman, Mehdi A. and Dai, Chenkai and Ahn, JoongHo and Fridman, Gene Y. and Della Santina, Charles C. (2013). "Multichannel Vestibular Prosthesis Employing Modulation of Pulse Rate and Current with Alignment Precompensation Elicits Improved VOR Performance in Monkeys". Journal of the Association for Research in Otolaryngology. 14: 233–248.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ a b c
Phillips, Christopher and DeFrancisci, Christina and Ling, Leo and Nie, Kaibao and Nowack, Amy and Phillips, James O. and Rubinstein, Jay T. (2013). "Postural responses to electrical stimulation of the vestibular end organs in human subjects". Experimental Brain Research. 229: 181–195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Fridman, Gene Y. and Della Santina, Charles C. (2013). "Safe Direct Current Stimulation to Expand Capabilities of Neural Prostheses". IEEE Trans Neural Syst Rehabil Eng. 21: 319–328.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ a b
Guyot, Jean-Philippe and Sigrist, Alain and Pelizzone, Marco and Kos, Maria I. (2011). "Adaptation to steady-state electrical stimulation of the vestibular system in humans". Annals of Otology, Rhinology & Laryngology. 120: 143–149.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ a b c
Harris, David M. and Bierer, Steven M. and Wells, Jonathon D. and Phillips, James O. (2009). "Optical nerve stimulation for a vestibular prosthesis". Processing of SPIE. 5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Della Santina, Charles C. and Migliaccio, Americo A. and Patel, Amit H. (2007). "A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-D vestibular sensation". IEEE transactions on bio-medical engineering. 54: 1016–1030.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ a b
Lumbreras, Vicente and Bas, Esperanza and Gupta, Chhavi and Rajguru, Suhrud M. (2014). "Pulsed Infrared Radiation Excites Cultured Neonatal Spiral and Vestibular Ganglion Neurons by Modulating Mitochondrial Calcium Cycling". Journal of Neurophysiology.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ a b
Dai, Chenkai and Fridman, Gene Y. and Della Santina, Charles C. (2011). "Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys". Hearing Research. 277: 204–210.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Holbrook EH, Puram SV, See RB, Tripp AG, Nair DG. (2019). "Induction of smell through transethmoid electrical stimulation of the olfactory bulb". Int Forum Allergy Rhinol. 2019, 9: 158–164.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑
Holbrook EH, Puram SV, See RB, Tripp AG, Nair DG. (2019). "Induction of smell through transethmoid electrical stimulation of the olfactory bulb". Int Forum Allergy Rhinol. 2019, 9: 158–164.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ a b Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. (June 2004). "A review of gas sensors employed in electronic nose applications". Sensor Review. 24 (2): 181–198. doi:10.1108/02602280410525977.
- ↑ Strauch, Martin; Lüdke, Alja; Münch, Daniel; Laudes, Thomas; Galizia, C. Giovanni; Martinelli, Eugenio; Lavra, Luca; Paolesse, Roberto; Ulivieri, Alessandra; Catini, Alexandro; Capuano, Rosamaria; Di Natale, Corrado (6 January 2014). "More than apples and oranges - Detecting cancer with a fruit fly's antenna". Scientific Reports. 4. doi:10.1038/srep03576.
Optogenetic Stimulation of Neurons
[edit | edit source]Photostimulation of neurons
[edit | edit source]Photo-stimulation of neurons is an emerging field of research. Neuronal firing is achieved by shining a focused light source onto the nerve cell, causing it to depolarize. There are two major ways to approach this goal: irradiation of the neurons with a laser, inducing a local temperature gradient; and the introduction of light sensitive channels or receptors into the nerve cell making it sensitive to light, similar to rods and cones in the human retina. Advantages over the traditionally used electric stimulation are increased precision and less to no tissue trauma.[1]
Electric vs Optic stimulation
[edit | edit source]Electric stimulation has inherent limitations compared to optic stimulation. To elicit reliable firing the electrodes have to be in physical contact with or in close proximity to the targeted tissue. Introduction of electrodes into the nerve tissue damages it and surrounding tissue.
In many cases the electrode array is introduced into electrically conductive tissue allowing for current spread, further decreasing the spacial resolution that can be achieved.
Measurement of the evoked neural activity is often contaminated by stimulation artefacts much larger than the measured neural activity. This is especially the case in measurements close to the excitation site.
In contrast, optic stimulation can reliably achieve excitation of single cells or small cell populations. It does not require direct contact to the target tissue, reducing tissue damage. Finally electrical recordings of neural response in close proximity are not contaminated by the excitation stimulus.[1] [2] [3] Although electrical stimulation suffers from the above mentioned drawbacks it is still the most well established and reliable method for nerve stimulation in patients.
Infrared Stimulation
[edit | edit source]Infrared stimulation is based on an infrared laser inducing a local temperature gradient inside the neuron. It does not require any modification of the cells prior to stimulation. The low energy laser does not cause damage to the tissue and elicits an artefact free stimulation. The exact mechanisms that lead to neuronal discharge are not known. However studies have shown that this phenomenon is is most likely due to local photothermal processes. Thus the IR irradiation creates a temperature gradient confined to a small space which rapidly vanishes after irradiation ceases. The local temperature rise of up to 9°C is thought to cause conformational changes in molecules ultimately leading to neuronal firing. At high irradiation frequencies the heat becomes additive, causing the irradiated tissue to heat up gradually and ultimately damaging the cell.[2][3]
Optogenetics
[edit | edit source]Optogenetics is the sensitisation of cells to light by the introduction of foreign genes, allowing temporal and spatial high resolution alteration of neural firing patterns. The genes can be expressed in genetic modification of animals or be introduced by vectors like viruses. Most light sensitizing genes used today were first discovered in unicellular organisms like algae or archaea. These genes can encode light sensitive ion channels or receptors producing various responses to optic stimulation.

For neuronal activation natural Channelrhodopsins (ChR) or engineered genetic variants thereof are commonly used. ChRs are light sensitive non-specific cation channels, which open when excited with blue light (480nm). In nerve cells ChR opening leads to sodium influx and membrane depolarisation.[4] [5] The light sensitive component is an all-trans-retinal which is also found in the human retina. Light induces a conformational change to 13-cis-retinal allowing cations to flow across the channel.[4][5][6][7] Introduction of specific point mutations close to the retinal binding site can alter the kinetic properties and specificity of the channel.[8] Linking ChR to other proteins allows for tools with diverse functionalities as in vivo monitoring of the introduced constructs.[9]
Halorhodopsin (HR) are light gated chloride ion pumps used for light activated neuronal inhibition. Optical excitation by yellow light (570nm) in sensitized neurons leads to an import of chloride ions and hyperpolarisation.[10][11] Like in ChR, the light sensitive molecule is also all-trans-retinal. Due to different stabilisation and thus wave length sensitivity differences of the retinal in HR and ChR, they can be used in the same cells and targeted separately. This allows for very close control of the activity in neural circuits.[11][12]
For optical control of cell pathways the Opto-XR proteins were developed,[13] where the X stands for the targeted signalling pathway. Opto-XRs consist of an animal-rhodopsin (bovine, rat etc.), with its intracellular domains exchanged for signalling sequences of the cell.[14] This allows optic regulation of the cells signalling pathway. The signaling sequences can either be activated or deactivated by conformational changes induced by light falling on the rhodopsin. This allows for specific activation of certain receptor pathways like serotonin or adrenergic signalling.[13][15]
Optic stimulation in Neuro-Prosthetics
[edit | edit source]Electric stimulation has long been used for evoking nerve firing in neuronal prosthetics. However, spread of electrical current and generation of electric fields limit the spatial resolution that can be achieved. This limits the fidelity of the transmitted signal.[16] In the case of auditory prostheses a maximum of around twenty electrodes is feasible, leaving the sound quality achieved far off the desired goal. A switch to optic based technology could achieve activation of smaller areas, increasing the amount of potentially perceived tones. Recent development in optic stimulation techniques promise ways to overcome those obstacles and improve prosthetic devices and the quality of live for patients.
Cochlear Implants
[edit | edit source]Infrared stimulation of the cochlea as well as the auditory nerve have been tested in various animal models such as rodents and cats. The optic variant shows remarkable precision concerning the area stimulated by the laser, which is approximately the same size as that activated by a medium loudness tone. It has also been shown that using low energy IR irradiation, constant stimulation can be achieved without gradual heating and damaging of the tissue. This enables the usage of the implant throughout the day without damaging the cochlear system. A major drawback of the IR stimulation is a much higher energy consumption compared to electrical stimulation.[2]
To overcome the energy problem described, researchers have begun to test an optogenetic approach in rodents. They genetically engineered mice to express Channelrhodopsins in spinal ganglion neurons. Sensitisation of the nerve cells reduced the energy required to induce firing compared to IR irradiation by a factor of seven (IR: 15 μJ, Optogen.: 2 μJ, Electric: 0.2 μJ). Stimulation was thus possible using μLEDs instead of lasers. In spite of this progress, implementation of this technology in humans in the near future is questionable. This is mainly due to the possible dangers of viral introduction of genetic material into an organism. Up to now only very few gene therapies have been approved. A save but still effective way of specifically infecting the cochlear organs would have to be implemented and approved.[17]
First patents have been registered describing potential optical cochlear implants for humans. These implants function similar to the traditional electrical implants. But instead of the electrodes they have VCSELs (vertical-cavity surface-emitting lasers) that are driven by the implant's input device. VCSELs are laser emitting diodes that can be fit into the small tubing of the implant. The lasers are directed at the organ of Corti and can be much closer spaced than electrodes, more than doubling the amount of implant output channels. Laser diodes are used for higher pitch signalling, while electrodes drive lower amplitude nerve cells.[18]
Vestibular Prostheses
[edit | edit source]Vestibular Prostheses aim to restore imbalance issues arising from dysfunction of the vestibular system. Since the semicircular canals are interconnected, current spread is a major problem in electrical stimulus delivery. Current spread can lead to additional stimulation of unwanted semicircular canals, resulting in incorrect balance signals sent to the brain. The possibility of using IR irradiation has been investigated. Irradiation of the ampullae did not evoke action potentials. The reason for failing stimulation might lie in an insensitivity of the hair cells to IR irradiation. Nevertheless, optical stimulation of the vestibular nerve might be feasible. It is as of yet unclear if separate stimulation of the nerves from different ampullae is possible in this manner.[2][19]
References
[edit | edit source]- ↑ a b Szobota, Stephanie; Isacoff, Ehud Y (2010). "Optical control of neuronal activity". Annual review of biophysics. 39: 329–348.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ↑ a b c d Richter, Claus-Peter; Matic, Agnella Izzo; Wells, Jonathon D; Jansen, E Duco; Walsh, Joseph T (2011). "Neural stimulation with optical radiation". Laser & photonics reviews. 5 (1): 68–80.
- ↑ a b Wells, Jonathon D; Cayce, Jonathan M; Mahadevan-jansen, Anita; Konrad, Peter E; Jansen, E Duco (2011). "Infrared Nerve Stimulation: A Novel Therapeutic Laser Modality". Optical-Thermal Response of Laser-Irradiated Tissue (2 ed.). Dordrecht: Springer Netherlands. pp. 915–939.
- ↑ a b Berthold, Peter; Tsunoda, Satoshi P; Ernst, Oliver P; Mages, Wolfgang; Gradmann, Dietrich; Hegemann, Peter (2008). "Channelrhodopsin-1 initiates phototaxis and photophobic responses in chlamydomonas by immediate light-induced depolarization". The Plant cell. 20 (6): 1665–1677.
- ↑ a b Nagel, Georg; Szellas, Tanjef; Huhn, Wolfram; Kateriya, Suneel; Adeishvili, Nona; Berthold, Peter; Ollig, Doris; Hegemann, Peter; Bamberg, Ernst (2003). "Channelrhodopsin-2, a directly light-gated cation-selective membrane channel". Proceedings of the National Academy of Sciences. 100 (24): 13940–13945.
- ↑ Bamann, Christian; Kirsch, Taryn; Nagel, Georg; Bamberg, Ernst (2008). "Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function". Journal of Molecular Biology. 375 (3): 686–694.
- ↑ Ernst, Oliver P; Sánchez Murcia, Pedro a; Daldrop, Peter; Tsunoda, Satoshi P; Kateriya, Suneel; Hegemann, Peter (2008). "Photoactivation of channelrhodopsin". The Journal of Biological Chemistry. 283 (3): 1637–1643.
- ↑ Gunaydin, Lisa; Yizhar, Ofer; Berndt, André; Sohal, Vikaas S; Deisseroth, Karl; Hegemann, Peter (2010). "Ultrafast optogenetic control". Nature neuroscience. 13 (3): 387–392.
- ↑ Lin, John Y; Lin, Michael Z; Steinbach, Paul; Tsien, Roger Y (2009). "Characterization of engineered channelrhodopsin variants with improved properties and kinetics". Biophysical journal. 96 (5): 1803–1814.
- ↑ Duschl, A; Lanyi, JK; Zimanyi, L (1990). "Properties and photochemistry of a halorhodopsin from the haloalkalophile, Natronobacterium pharaonis". Journal of Biological Chemistry. 265: 1261–1267.
- ↑ a b Zhang, Feng; Wang, Li-Ping; Brauner, Martin; Liewald, Jana F; Kay, Kenneth; Watzke, Natalie; Wood, Phillip G; Bamberg, Ernst; Nagel, Georg; Gottschalk, Alexander; Deisseroth, Karl (2007). "Multimodal fast optical interrogation of neural circuitry". Nature. 446 (7136): 633–639.
- ↑ Han, Xue; Boyden, Edward S (2007). "Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution". Plos one. 2 (3): e299.
- ↑ a b Kim, Jong-myoung; Hwa, John; Garriga, Pere; Reeves, Philip J; Rajbhandary, Uttam L; Khorana, H Gobind (2005). "Light-Driven Activation of 2 -Adrenergic Receptor Signaling by a Chimeric Rhodopsin Containing the 2 -Adrenergic Receptor Cytoplasmic Loops". Biochemistry. 44 (7): 2284–2292.
- ↑ Airan, Raag D; Thompson, Kimberly R; Fenno, Lief E; Bernstein, Hannah; Deisseroth, Karl (2009). "Temporally precise in vivo control of intracellular signalling". Nature. 458 (7241): 1025–1029.
- ↑ Oh, Eugene; Maejima, Takashi; Liu, Chen; Deneris, Evan; Herlitze, Stefan (2010). "Substitution of 5-HT 1A Receptor Signaling by a Light-activated G Protein-coupled Receptor". The Journal of Biological Chemistry. 285 (40): 30825–30836.
- ↑ McGill, K C; Cummins, K L; Dorfman, L J; Berlizot, B B; Leutkemeyer, K; Nishimura, D G; Widrow, B (1982). "On the nature and elimination of stimulus artifact in nerve signals evoked and recorded using surface electrodes". IEEE transactions on bio-medical engineering. 29 (2): 129–137.
- ↑ Hernandez, VH; Gehrt, Anna; Reuter, Kirsten; Jing, Zhizi; Jeschke, Marcus; Schulz, Alejandro Mendoza; Hoch, Gerhard; Bartels, Matthias; Vogt, Gerhard; Garnham, Carolyn W.; Yawo, Hiromu; Fukazawa, Yugo; Augustine, George J.; Bamberg, Ernst; Kügler, Sebastian; Salditt, Tim; Hoz, Livia de; Strenzke, Nicola; Moser, Tobias (2014). "Optogenetic stimulation of the auditory pathway". The Journal of Clinical Investigation. 124 (3): 1114–1129.
- ↑ OPTICAL-STIMULATION COCHLEAR IMPLANT WITH ELECTRODE(S) AT THE APICAL END FOR ELECTRICAL STIMULATION OF APICAL SPIRAL GANGLION CELLS OF THE COCHLEA, Jan. 24, 2013
{{citation}}: Check date values in:|publication-date=(help); Unknown parameter|country-code=ignored (help); Unknown parameter|inventor-first=ignored (help); Unknown parameter|inventor-last=ignored (help); Unknown parameter|inventor2-first=ignored (help); Unknown parameter|inventor2-last=ignored (help); Unknown parameter|issue-date=ignored (help); Unknown parameter|patent-number=ignored (help) - ↑ Harris, DM; Bierer, SM (2009). "Optical nerve stimulation for a vestibular prosthesis". Proceedings of SPIE. 5: 71800R.

