Proteomics/Protein Identification - Mass Spectrometry/Instrumentation
This Section:
How does a Mass Spectrometer work?
[edit | edit source]
A mass spectrometer is made up of three components: an ion source, mass analyzer, and a detector. The unknown sample which may originate as solid, liquid, solution or vapor, is presented to the ionization source. After ionizing the sample, the ions of the sample are passed to the mass analyzer region where separation based on the mass-to-charge ratio occurs. Once separated by the analyzer, the ions then enter the detector portion of the mass spectrometer. At this point, the machine calculates the mass-to-charge ratio and the relative abundance of each of the different ions. From this information, a spectrum graph can be created such as the one to the right.
Most mass spectrometers are maintained under a vacuum to improve the chances of ions traveling from ionization source to detector without interference by collision with air molecules.
Ion Source
[edit | edit source]The ion source is the mass spectrometer component which ionizes the sample to be analyzed. Ionization mainly serves to present the sample as vaporized ions which can be acted upon by the mass analyzer and measured by the ion detector. There are many different methods available to ionize samples, such as positive or negative ion modes. The ionization method chosen should depend on the type of sample and the type of mass spectrometer.
Ionization Methods:

There are three types of ionization methods, electron ionization, chemical ionization and photo-induced ionization. Electron ionization involves application of an electrical current to the sample to induce ionization. Chemical ionization involves interaction of the sample with reagent molecules to induce ionization. Ions produced are often denoted with symbols that indicate the nature of the ionization, eg. [M+H]+ is used to represent a molecule which is protonated.
- Electron ionization
- Chemical Ionization
The methods commonly used in proteomics are ‘Matrix Assisted Laser Desorption Ionization’ or MALDI and ‘Electrospray Ionization’ also known as ESI. Atmospheric pressure chemical ionization and Atmospheric pressure photo-ionization are two other forms as well.
MALDI uses a solid support target plate where a UV active matrix (solid or liquid) is spotted on the plate followed by the sample over the matrix. The laser hits the spot on the crystallized matrix and transfers energy from the matrix molecule to the sample. This energy transfer vaporizes the sample, sending a plume of ions into the MALDI source. This plume of ions is then collected and held in the source until a pulse sends them all out simultaneously. If the MALDI is attached to a Time of Flight(TOF) mass analyzer these ions are then sent down the TOF tube (typically ~2m) and are separated according to their velocity (light ions hitting first). MALDI is the preferred instrumentation for proteomics due to ease of reading the spectrum; most ions are found in the +1 charge state [M+H].
Electrospray, on the other hand, is done by injecting the sample dissolved in a slightly acidic solution through a heated capillary that has a voltage around 10v. This allows for highly charged particles to be formed at the tip of the capillary. As the particles evaporate, their charge/volume increases to a point where charge repulsion forces take over and the particle will explode. A small drop will form which continues the process until individual molecules are in the gas phase and charged. These ions will then travel into the analyzer, typically a quadrupole, to be scanned one mass at a time. The molecules in electrospray tend to be multiply charged and even though the upper mass limit of a quadrupole is 2000 m/z, the multiple charges allow for high mass ions or proteins to be identified. Proteins will have a charge envelope in which each peak has a different amount of charges on them. Special software is needed to deconvolve the multiple charge species peaks into a single mass peak, such as MassLynx from Waters. This form of ionization is good for most compounds, although is not best with neutral or low polarity molecules.
Atmospheric pressure chemical ionization involves interaction of the sample with reagent molecules to induce ionization. As in electrospray ionization, liquid is pumped through a capillary. At the tip it is nebulized and a corona discharge takes place ionizing the molecules. The molecules interact with the analyte and transfer their charge. This form of ionization is good for small thermally stable molecules.
Atmospheric pressure photo-ionization uses photons to excite and ionize the molecules after they have been nebulized. This form of ionization is good for neutral compounds.
Mass Analyzers
[edit | edit source]The mass analyzer is the component that separates the ions created from the ion source by their mass-to-charge ratios. Mass analyzers are based on the principles of charged particles in an electric or magnetic field. By using Lorentz force law and Newton’s second law of motion you can generate the following equation:
Where m is the mass, the ionic charge is q , a the acceleration, E is the electric field, and the vector cross product of the ion velocity and the magnetic field is v x B. This equation says that two particles with the same mass to charge ratio (m/q) will behave exactly the same.
So what this equation is basically saying is that the mass to charge ratio acts as a determinant of acceleration of the ion, which can also be represented as the addition of the electric field plus the cross product of the ion velocity and magnetic field.
Scanning Mass Analyzers
[edit | edit source]Scanning mass analyzers need to separate the ions based on their relative abundances and mass to charge ratios. Electromagnetic fields are used to separate the ions based on their mass to charge ratios, by using a slit they are able to regulate which mass to charge ratio ions get to the detector. Once selected for a particular mass to charge ratio, the ion current is then recorded as a function of time which is analagous to mass.
Sector Mass Spectrometer
[edit | edit source]Mass spectrometer that uses a mass analyzer using magnetic, electric or static sector in it is called a sector instrument. This also works in combination of sectors like BEB, magnetic-electric-magnetic. In the present days, these sector instruments are mostly double focusing i.e, the ion beams are focused in both direction and velocity. Here is how a magnetic sector mass spectrometer works, imagine a tube like thing between two electromagnets. When the electrons are passed through the tube from one end to another the magnetic field bends the electron stream by exerting turning force on it. Then m/z ratio is determined.
Orbitrap Mass spectrometer
[edit | edit source]Orbitrap Mass spectrometer is the one in which ions are subjected to an electric field produced by electrodes tangentially. Also these ions are trapped between outer electrodes, where as ions are attracted with an electrostatic attraction to the inner electrode which is balanced by centrifugal force. Therefore these ions move in a circular manner around the inner electrode also back and forth with a particular m/z ratio. By using this ion oscillation based on the m/z ratio, the trap can act as a mass analyzer.
Quadrupole Mass Spectrometers
[edit | edit source]You can think of this method as a filter or funnel which only allows certain ion masses to pass through. The "funnel" is actually a combination of positively and negatively charged metal rods which together form a channel through which the ions travel. The theory is that only selected masses will be able to pass through the channel, as all other ions won't have a stable trajectory though it and will hit the quadrupole rods, stopping the ion from reaching the detector.
Ion trap Quadrupole Spectrometer
[edit | edit source]A quadrupole ion trap mass spectrometer consists of hyperbolic electrodes with a ring and two endcaps, which is the core of this instrument. In this method, ions are trapped and then sequentially ejected into a conventional electron multiplier detector from the ion trap. That way all ions can be stored during the process of mass analysis. Recent findings showed that using 1 mtorr of helium gas in the trapping volume substantially improved the resolution of the instrument with the kinetic energy of the ions reducing and the ion trajectories contracted to the center of the trap. A packet can be formed with a given m/z ions. This spectrometer is used widely for commercial purposes because of the high resolution and it is inexpensive.
Linear Quadrupole Mass Spectrometer
[edit | edit source]In the linear ion trap mass spectrometer the ions are trapped within a set of quadrupole rods to hold the ions radially and end electrodes to maintain the ions axially with a static electrical potential. It is simply said that unlike Quadrupole Ion Trap, which uses a 3-D field, Linear Quadrupole Ion Trap uses a 2-D field. It has a selective mass filter that detects the ions of particular m/z ratio. This method has advantages like higher ion storage capacity and faster scanning technique.
Time of Flight Mass Analysers
[edit | edit source]The TOF (Time of Flight) is a mass analyzer that allows ions to flow down a field free region; which allows the ions with a greater velocity, lighter ions, to hit the detector first. This is especially compatible with MALDI due to the fact that the TOF needs a pulsed instrument for its source. In this way ions are generated in the MALDI source and held there for a brief time and all are pulsed into the TOF at the same exact time. In this way, If all ions have the same kinetic energy, the ions with the lower mass will have a higher velocity and reach the detector first; whereas the ions with the higher mass will have a lower velocity and hit the detector last.
The kinetic energy of an ion leaving a source if given by:
Where velocity v is defined by the Length of the path L divided by time t.
By substituting this equation into the first and solving for time you arrive at.
From this equation you can easily see how mass directly affects travel time. Mass is directly proportional to time. Using the m/e portion of the equation you can clearly see how a larger mass means a longer time, and likewise how a lower mass would mean a smaller m/e and thus shorter travel time.
Ion Cyclotron Resonance Spectrometer
[edit | edit source]ICR is a form of trapped Ion mass analyzer, that specifically is defined as a static trap. It is basically a box with three parallel metal sides. Trapped ion analyzers work by keeping the ions in the trap and controlling the ions by using positive and negatively charged electrical fields in a carefully series of timed events.
ICR specifically works on the principle that in a magnetic field, ions move in a circular path whose frequency is mass dependent. So using the cyclotron frequency you can surmise the mass. Equating the Lorentz force in a magnetic field to the equation for centripetal force yields.
You can then easily solve the above equation for the frequency F or
Groups of ions with the same mass to charge ratios will have the same cyclotron frequency but may be moving out of synch with one another, this is why an excitation pulse is needed to bring the resonant ions into phase with one another and the excitation pulse. Next the ions which will be passing close to the ICR cell receiver plates cause "image currents" which can be collected and amplified and analyzed. This signal registered in the receiver plates depends both on the number of ions and their distances from the receiver plates.
Fourier transformation
[edit | edit source]This mass spectrometry includes a mass analyzer that uses cyclotron frequency of the ions to determine their m/z ratio. Here a magnetic field with electric trapping plates known as penning trap is used to hold the ions. An oscillating electric field with the magnetic field at right angle to it is used to excite the trapped ions to a larger cyclotron radius. This results in packets of oscillating ions. The trapping plates detects the signal as a image current and results in a interferogram with sine waves called free induction decay (FID). By performing a Fourier transform to this data, a mass spectrum can be generated with useful signal.
Detector
[edit | edit source]Those ions which pass through analyzer are now separated by the desired methods. This mass spectrometry component records the charge induced by an ion passing by a surface or current produced when an ion hits a surface. From these charges or currents, a mass spectrum can be produced as well as measure the total number of ions at each each m/z which are present. Due to the fact that the number of ions entering the detector at any given moment is minuscule, signal amplification is often necessary.
Next section: Types of Mass Spectrometry
References
[edit | edit source]- Experimental Techniques: MALDI TOF, http://www.microbiology.science.ru.nl/tech/malditof/
- An Introduction to Mass Spectrometry, http://www.astbury.leeds.ac.uk/facil/MStut/mstutorial.htm
- Electron Ionization Sources: The Basics, http://www.spectroscopymag.com/spectroscopy/article/articleDetail.jsp?id=358670
- What other techniques are used to produce ions?, http://www.asms.org/whatisms/p11.html
- Magnetic Sector Mass Spectrometry, http://www.wooster.edu/Chemistry/analytical/ms/default.html
- http://en.wikipedia.org/wiki/Orbitrap
- The Whys and Wherefores of Quadrupole Ion Trap Mass Spectrometry, http://www.abrf.org/ABRFNews/1996/September1996/sep96iontrap.html
- http://en.wikipedia.org/wiki/Quadrupole_ion_trap
- Fourier Transform Ion Cyclotron Resonance theory: a brief review, http://www.emsl.pnl.gov/docs/msd/mass_spec/home/fticrtut.html
- Fourier Transform Mass Spectrometry, http://www.bumc.bu.edu/Dept/Content.aspx?DepartmentID=385&PageID=7205
Articles Summarized
[edit | edit source]Principles and Applications of Liquid Chromatography-Mass Spectrometry in Clinical Biochemistry
[edit | edit source]Pitt JJ. The Clinical Biochemist Reviews Volume:3019-34 (2009)
Main Focus
[edit | edit source]One benefit of Liquid Chromatography - Mass Spectrometry is there are different configurations to allow for results more geared toward the experiment.
Summary
[edit | edit source]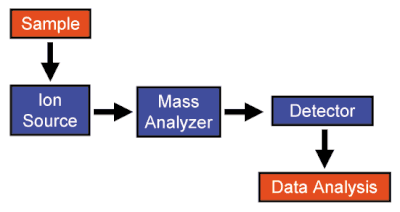
LC-MS was slow to take off due to inefficient technologies. However, in the mid 1990’s, after new technologies were developed, it became more popular. This was in part due to its high specificity and ability to handle more complex mixtures than the other options available at the time, such as GC-MS. Mass spectrometry starts by ionizing samples to generate charged molecule fragments then analyzing their mass to charge-ratio. There are different technologies used to perform the mass spectrometry part of LC-MS to obtain different results, some being more versatile than others, such as changing the ion source, changing the mass analyzer, changing the ion suppression as well as changing the direct injection method.
Different ion sources that can be used clinically would be an electrospray ionization source, an atmospheric pressure chemical ionization source or an atmospheric pressure photo-ionization source. Electrospray ionization, also known as ESI, works well on polar molecules; metabolites, xenobiotics and peptides are some examples. Atmospheric pressure chemical ionization source, also known as APCI, is great analyzer for small thermally stable molecules and neutral non-polar molecules such as free steroids. Atmospheric pressure photo-ionization, also known as APPI, works well with neutral compounds such as steroids.
There are four different types of mass analyzers that can be used; these are the quadrupole analyzers, time-of-flight analyzers, ion trap analyzers, and hybrid analyzers. Quadrupole analyzers are are widely used because their ease of scanning and good quality quantitative data. Time-of-flight analyzers are used for small molecules because of its high sensitivity. Ion trap analyzers have the ability to fragment an ionize ions several times giving so-called MSn capabilities. Hybrid analyzers can be switched between ion trap mode and conventional quadrupole mode.
There are also different steps that can be changed in the Liquid Chromatography part of LC-MS to make the procedure more versatile such as changing the flow rate, the mobile phase, the resolution and through-put and the quantitation (calibration). The parameters and conditions involved in LC-MS can be changed to optimize the assay. However, these conditions are specific to the particular analyte and the LC separation. Therefore there are no general conditions. Some of these conditions include using a dilute solution of the analyte and using single MS or tandem MS. It is also important to make sure the LC-MS system is working properly with protocols to detect deviations from normal performance. LC-MS is used in the Biochemical screening for genetic disorders as well as therapeutic drug monitoring and steroid hormones.
New Terms
[edit | edit source]- GC-MS
- Gas chromatography-mass spectrometry (GC-MS) is a method that combines the features of gas-liquid chromatography and mass spectrometry to identify different substances within a test sample. ( http://en.wikipedia.org/wiki/GC-MS)
- Ion source
- A device in which gas ions are produced, focused, accelerated, and emitted as a narrow beam. Also known as ion gun; ionization source. ( http://www.answers.com/topic/ion-source )
- Calibration
- a set of graduations to indicate values or positions —usually used in plural ( http://www.merriam-webster.com/dictionary/calibration )
- Deviation
- noticeable or marked departure from accepted norms of behavior ( http://www.merriam-webster.com/dictionary/deviation )
- Xenobiotics
- a chemical compound (as a drug, pesticide, or carcinogen) that is foreign to a living organism ( http://www.merriam-webster.com/dictionary/xenobiotics )
Course Relevance
[edit | edit source]- How is this applicable to the proteomics class?
It is important to be able to analyze what makes up a protein so that we can understand the affects of that protein on an organism. Being able to see the differences between a given protein and its normal counterpart could help in the understanding of genetic diseases. LC-MS gives scientists the ability do protein separation.
Websites Summarized
[edit | edit source]What is Mass Spectrometry?
[edit | edit source]Chiu CM, Muddiman DC. http://www.asms.org/whatisms/index.html (3/28/09)
Main Focus
[edit | edit source]This website discusses what mass spectrometry is, the history behind it, and how it works.
Summary
[edit | edit source]Mass spectrometry is a powerful analytical technique that is used to identify unknown compounds, to quantify known compounds, and to elucidate the structure and chemical properties of molecules. Compounds can be identified at very low concentrations. Mass spectrometry is used by a wide range of professionals such as physicians, astronomers, and biologists. For example it can monitor the breath of patients by anesthesiologists during surgery and determine the composition of molecular species found in space. It can be used to identify structures of biomolecules as well sequence them. It is also used to identify and quantitate compounds of complex organic mixtures. Mass spectrometry was invented by J.J. Thomson in a vacuum tube. His invention was used to discover a number of isotopes, to determine the relative abundance of the isotopes, and to measure their "exact masses" which are important in the foundation for later developments in diverse fields ranging from geochronology to biochemical research.
A mass spectrometer is an instrument that measures the masses of individual molecules that have been converted into ions by becoming electrically charged. A mass spectrometer does not actually measure the molecular mass directly, but rather the mass-to-charge ratio of the ions formed from the molecules. The charge on an ion is denoted by the integer number of the fundamental unit of charge, and the mass-to-charge ratio. It represents daltons per fundamental unit of charge. Results are in a generated mass spectrum. A mass spectrum is a graph of ion intensity as a function of mass-to-charge ratio.
New Terms
[edit | edit source]- dalton
- a unit of mass for expressing masses of atoms, molecules, or nuclear particles equal to 1⁄12 of the atomic mass of the most abundant carbon isotope ( http://www.merriam-webster.com/medical/dalton )
- corona
- a faint glow adjacent to the surface of an electrical conductor at high voltage ( http://www.merriam-webster.com/dictionary/corona )
- mass spectrum
- the spectrum of a stream of gaseous ions separated according to their differing mass and charge ( http://www.merriam-webster.com/dictionary/mass%20spectrum )
- elucidate
- to make lucid especially by explanation or analysis ( http://www.merriam-webster.com/dictionary/elucidate )
- isotopes
- any of two or more species of atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with differing atomic mass or mass number and different physical properties ( http://www.merriam-webster.com/dictionary/isotopes )
Course Relevance
[edit | edit source]- How is this applicable to the proteomics class?
Proteomics is the study of the protein and is defined as the qualitative and quantitative comparison of proteomes under different conditions to further unravel biological processes. Mass spectrometry allows for the study of the components of an organism and how they create their individual functions.







