Organic Chemistry/Aromatic reactions
<< Aromatics | Aromatic reactions | Ketones and aldehydes>>
The lack of reactivity of arenes is notable when compared to the reactivities of typical compounds containing multiple conjugated double bonds. For example, 1,3,5-hexatriene is much more reactive than hexane, hexene, or any hexadiene. Benzene is much less reactive than any of these. Any of the alkenes will be readily converted to alcohols in the presence of a dilute aqueous solution of H2SO4, but benzene is inert. Similarly, alkenes react readily with halogens and hydrogen halides by addition to give alkyl halides, whereas halogens react with benzene by substitution and only in the presence of a catalyst. KMnO4 or chromic acid solutions (typically CrO3 or K2Cr2O7) cleave the double bonds of alkenes, giving ketones or carboxylic acids, but do not react at all with benzene. Because of the stability of aromatic compounds, however, reactions involving these have extremely high activation energies, for passage to the transition state necessarily requires disruption of the aromatic system, resulting in a temporary loss of aromatic stabilization energy. Instead of reacting by addition and elimination, as nonaromatic compounds often do, benzene and its derivatives usually react by electrophilic aromatic substitution.
Redox
[edit | edit source]Birch reduction
[edit | edit source]The Birch reduction[1] is the reduction of aromatic compounds by sodium in liquid ammonia. It is attributed to the chemist Arthur Birch. The reaction product is a 1,4-cyclohexadiene. The metal can also be lithium or potassium and the hydrogen atoms are supplied by an alcohol such as ethanol or tert-butanol.

The first step of a Birch reduction is a one-electron reduction of the aromatic ring to a radical anion. Sodium is oxidized to the sodium ion Na+. This intermediate is able to dimerize to the dianion. In the presence of an alcohol the second intermediate is a free radical which takes up another electron to form the carbanion. This carbanion abstracts a proton from the alcohol to form the cyclohexadiene.

In the presence of an alkyl halide, the carbanion can also engage in nucleophilic substitution with carbon-carbon bond formation. In substituted aromatics an electron withdrawing substituent such as a carboxylic acid stabilizes a carbanion and the least-substituted alkene is generated. With an electron donating substituent, the opposite effect occurs. The non-conjugated 1,4-addition product is preferred over the conjugated 1,3-diene which is explicable by the principle of least motion. Experimental alkali metal alternatives that are safer to handle, such as the M-SG reducing agent, also exist.
Oxidation of Benzene in the Human Body
[edit | edit source]Because benzene is nonpolar, it cannot be passed in urine, and will remain in the body until oxidized. Benzene itself is not dangerous to health, but in order to be passed, it is oxidized by cytochrome P-450 in the liver. This produces benzene oxide, a highly teratogenic and carcinogenic compound. Benzene has been replaced by toluene as an industrial solvent, because toluene can be oxidized to benzoic acid, which is mostly harmless to health, and is quickly passed. The decomposition of benzoic acid into benzene and carbon dioxide in soda pop has become an issue recently.
Nucleophilic Aromatic Substitution
[edit | edit source]A nucleophilic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide on an aromatic ring. In order to understand this type of reaction, it is important to recognize which chemical groups are good leaving groups and which are not.
Leaving Groups
[edit | edit source]A leaving group can probably most simply be described as an atom or molecule that detaches from an organic molecule. The ability for a functional group to leave is called lability. Leaving groups affect the intrinsic reactivity of the molecule as a whole, but only until, quite naturally, they actually leave.
The lower the pKa of the conjugate acid for a given leaving group, the better that leaving group is at actually leaving. This is because such groups can easily stabilize any developing negative charge and without stabilization, a leaving group will actually become a nucleophile causing the reaction to cycle pointlessly between attached and detached forms. (This explains why a strong base is nearly always a poor leaving group.)
In room temperature water, the sequence of lability is:
- Less lability
- amine/amide (NH2-)
- alkoxy/alkoxide (RO-)
- hydroxyl/hydroxide (HO-)
- carboxylate (RCOO-)
- fluoro/fluoride (F-)
- water (H2O)
- chloro/chloride (Cl-)
- bromo/bromide (Br-)
- iodo/iodide (I-)
- azide (N3-)
- thiocyanate (SCN-)
- nitro/nitrite (NO2)
- cyano/cyanide (CN-)
- Greater Lability
Rate of Reaction
[edit | edit source]The better a leaving group, the faster a nucleophilic reaction will occur. This is demonstrated by comparisons of the kinetics between halogenalkanes, where the bromides dissociate more quickly than the chlorides, but the iodides dissociate more rapidly than either of the other two. This is because the bond between the halogen and its nearest carbon must be broken at some point for a nucleophilic substitution to take place. A bond between iodine and carbon is far more polarizable than a bond between carbon and chlorine, for example, due to iodine's relatively large size and relatively large number of ionizable electrons. The fact that water is a far better leaving group than hydroxide also has the important consequence that the rate of a reaction in which hydroxide leaves is increased dramatically by the presence of an acid, for hydroxide is then protonated to water, a much weaker nucleophile.
Types of Reactions
[edit | edit source]There are three nucleophilic substitution mechanisms commonly encountered with aromatic systems, the SNAr (addition-elimination) mechanism, the benzyne mechanism and the free radical SRN1 mechanism. The most important of these is the SNAr mechanism, where electron withdrawing groups activate the ring towards nucleophilic attack, for example if there are nitro functional groups positioned ortho or para to the halide leaving group. It is not generally necessary to discuss these types in detail within the context of an introductory organic chemistry course.
Electrophilic Aromatic Substitution
[edit | edit source]Electrophiles are particles with a deficiency of electrons. Therefore they are likely to react with substances that have excess electrons. Aromatic compounds have increased electron density in the form of delocalized π-orbitals.
Step 1: Formation of a π-complex
[edit | edit source]At first, the electrophile interacts with the delocalized orbitals of the aromatic ring and a π-complex is formed.
No chemical bonds are formed at this stage. Evidence of the formation of a π-complex as an intermediate state has been found for some reactions, but not for all, since the chemical interaction in π-complexes is very weak.
Step 2: Formation of a σ-complex
[edit | edit source]After the π-complex is formed, in the presence of an electron acceptor another complex is formed - the σ-complex. It is a cationic species, an intermediate that lacks aromatic properties, but its four π-electrons are delocalized across the ring, which stabilizes the cation somewhat, sometimes allowing its isolation. An example would be the salt mesityl fluoroborate, which is stable at low temperatures, and is prepared by the reaction of mesitylene (1,3,5-trimethylbenzene) with fluoroboric acid (BF3/HF); the cation of this salt is protonated mesitylene. σ-complexes are also known as Wheland intermediates.
Step 3: Formation of a Substituted product
[edit | edit source]At the next stage the σ-complex decomposes, freeing a hydrogen cation and forming the product of substitution.
Electrophilic aromatic halogenation
[edit | edit source]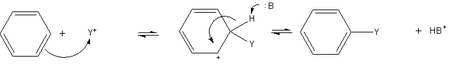
Another important reaction of benzene is the electrophilic substitution of halides, a specific type of electrophilic aromatic substitution. These reactions are very useful for adding substituents to an aromatic system. The rates of the reactions increase with the electrophilicity of the halogen: hence, fluorination in this manner is too rapid and exothermic to be practical, whereas iodine requires the most vigorous conditions. Chlorination and bromination are the most often practiced in the lab of the four possible halogenations. Halobenzenes are used for pesticides, as well as the precursors to other products. Many COX-2 inhibitors contain halobenzene subunits.
Some highly activated aromatic compounds, such as phenol and aniline, are reactive enough to undergo halogenation without a catalyst, but for typical benzene derivatives (and benzene itself), the reactions are extremely slow at room temperature in the absence of a catalyst. Usually, Lewis acids are used as catalysts, which work by helping to polarize the halogen-halogen bond, thus decreasing the electron density around one halogen atom, making it more electrophilic. The most common catalysts used are either Fe or Al, or their respective chlorides and bromides (+3 oxidation state). Iron(III) bromide and iron(III) chloride lose their catalytic activity if they are hydrolyzed by any moisture present, including atmospheric water vapor. Therefore, they are generated in situ by adding iron fillings to bromine or chlorine. Iodination is carried out under different conditions: periodic acid is often used as a catalyst. Under these conditions, the I+ ion is formed, which is sufficiently electrophilic to attack the ring. Iodination can also be accomplished using a diazonium reaction. Fluorination is most often done using this technique, as the use of fluorine gas is inconvenient and often fragments organic compounds.
Halogenation of aromatic compounds differs from the additions to alkenes or the free-radical halogenations of alkanes, which do not require Lewis acid catalysts. The formation of the arenium ion results in the temporary loss of aromaticity, the overall result being that the reaction's activation energy is higher than those of halogenations of aliphatic compounds.
Halogenation of phenols is faster in polar solvents due to the dissociation of phenol, because the phenoxide (-O-) group is more strongly activating than hydroxyl itself.
Electrophilic aromatic sulfonation
[edit | edit source]Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution.
The electrophile of such a reaction is sulfur trioxide (SO3), which can be released from oleum (also known as fuming sulfuric acid), essentially sulfuric acid in which gaseous sulfur trioxide has been dissolved.
In contrast to aromatic nitration and other electrophilic aromatic substitutions, aromatic sulfonation is reversible. Sulfonation takes place in strongly acidic conditions, and desulfonation can occur on heating with a trace of acid. This also means that thermodynamic, rather than kinetic, control can be achieved at high temperatures. Hence, directive effects are not expected to play a key role in determining the proportions of isomeric products of high-temperature sulfonation.
Aromatic sulfonic acids can be intermediates in the preparation of dyes and many pharmaceuticals. Sulfonation of aniline produces p-aminobenzenesulfonic acid or sulfanilic acid, which is a zwitterionic compound with an unusually high melting point. The amide of this compound and related compounds form a large group of sulfa drugs (a type of antibiotic).
Overall reaction: ArH + SO3 → ArSO3H
Electrophilic aromatic nitration
[edit | edit source]Nitration occurs with aromatic organic compounds via an electrophilic substitution mechanism involving the attack of the electron-rich benzene ring by the nitronium (nitryl) ion. Benzene is commonly nitrated by refluxing with a mixture of concentrated sulfuric acid and concentrated nitric acid at 50°C. The sulfuric acid is regenerated and hence acts as a catalyst.
Selectivity is always a challenge in nitrations. Fluorenone nitration is selective and yields a tri-nitro compound or tetra-nitro compound by tweaking reaction conditions just slightly. Another example of trinitration can be found in the synthesis of phloroglucinol. Other nitration reagents include nitronium tetrafluoroborate which is a true nitronium salt. This compound can be prepared from hydrogen fluoride, nitric acid and boron trifluoride. Aromatic nitro compounds are important intermediates for anilines; the latter may be readily prepared by action of a reducing agent.
Overall reaction: ArH + HNO3 → ArNO2 + H2O
Friedel-Crafts alkylation
[edit | edit source]
The Friedel-Crafts reactions, discovered by French alkaloid chemist Charles Friedel and his American partner, James Crafts, in 1877, is either the alkylation or acylation of aromatic compounds catalyzed by a Lewis acid. They are very useful in the lab for formation of carbon-carbon bonds between an aromatic nucleus and a side chain.
Source of electrophile
[edit | edit source]Friedel-Crafts alkylation is an example of electrophilic substitution in aromatic compounds. The electrophile is formed in the reaction of an alkyl halide with a Lewis acid. The Lewis acid polarizes the alkyl halide molecule, causing the hydrocarbon part of it to bear a positive charge and thus become more electrophilic.
CH3—Cl + AlCl3 → CH3+ + AlCl4−
or
CH3Cl + AlCl3 → CH3δ+Cl+Al−Cl3
(The carbon atom has a slight excess of positive charge, as the electronegative chlorine atom draws electron density towards itself. The chlorine atom has a positive charge, as it has formed a sub-ordinate bond with the aluminium atom. In effect, the Cl atom has lost an electron, while the Al atom has gained an electron. Therefore, the Al atom has a negative charge.)
Mechanism of alkylation
[edit | edit source]The polarized, electrophilic molecule then seeks to saturate its electron deficiency and forms a π-complex with the aromatic compound that is rich in π-electrons. Formation a π-complex does not lead to loss of aromaticity. The aromaticity is lost however in the σ-complex that is the next stage of reaction. The positive charge in the σ-complex is evenly distributed across the benzene ring.
C6H6 + CH3+ → C6H6+Br → C6H5Br + H+
The σ-complex C6H6+Br can be separated (it is stable at low temperatures), while the π-complex can not.
Restrictions
[edit | edit source]- Deactivating functional groups, such as nitro (-NO2), usually prevent the reaction from occurring at any appreciable rate, so it is possible to use solvents such as nitrobenzene for Friedel-Crafts alkylation.
- Primary and secondary carbocations are much less stable than tertiary cations, so rearrangement typically occurs when one attempts to introduce primary and secondary alkyl groups onto the ring. Hence, Friedel-Crafts alkylation using n-butyl chloride generates the n-butylium cation, which rearranges to the t-butyl cation, which is far more stable, and the product is exclusively the t-butyl derivative. This may, in some cases, be circumvented through use of a weaker Lewis acid.
- The Friedel-Crafts reaction can not be used to alkylate compounds which are sensitive to acids, including many heterocycles.
- Another factor that restricts the use of Friedel-Crafts alkylation is polyalkylation. Since alkyl groups have an activating influence, substituted aromatic compounds alkylate more easily than the original compounds, so that the attempted methylation of benzene to give toluene often gives significant amounts of xylene and mesitylene. The usual workaround is to acylate first (see the following sections) and then reduce the carbonyl group to an alkyl group.
Friedel-Crafts acylation
[edit | edit source]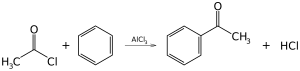
Friedel-Crafts acylation, like Friedel-Crafts alkylation, is a classic example of electrophilic substitution.
Source of electrophile
[edit | edit source]Reacting with Lewis acids, anhydrides and chloranhydrides of acids become strongly polarized and often form acylium cations.
RCOCl + AlCl3 → RC+O + AlCl4-
Mechanism of acylation
[edit | edit source]The mechanism of acylation is very similar to that of alkylation.
C6H6 + RC+O → C6H6—CO—R + H+
The ketone that is formed then forms a complex with aluminum chloride, reducing its catalytic activity.
C6H6—CO—R + AlCl3 → C6H6—C+(R)—O—Al−Cl3
Therefore, a much greater amount of catalyst is required for acylation than for alkylation.
Restrictions
[edit | edit source]- Although no isomerisation of cations happens, due to the reasonance stabilization provided by the acylium ion, certain cations may lose CO and alkylation will occur instead of acylation. For example, an attempt to add pivalyl (neopentanoyl) to an aromatic ring will result in loss of CO from the cation, which then results in the t-butyl derivative being formed.
- Acidophobic aromatic compounds, such as many heterocycles can't exist in the presence of both Lewis acids and anhydrides.
- Formyl chloride is unstable and cannot be used to introduce the formyl group onto a ring through Friedel-Crafts acylation. Instead, the Gattermann-Koch reaction is often used.
Applications
[edit | edit source]Friedel-Crafts acylation is used, for example, in the synthesis of anthraquinone from benzene and phtalic anhydride.
In laboratory synthesis Friedel-Crafts acylation is often used instead of alkylation in cases where alkylation is difficult or impossible, such as synthesis of monosubstituted alkylbenzenes.
External links
[edit | edit source]- reduction of o-anisic acid to 2-heptyl-2-hexenone in Organic Syntheses Article
- reduction of naphthalene to 1,4,5,8-Tetrahydronaphthalene (isotetralin) in Organic Syntheses Article.
- reduction of o-xylene to 1,2-Dimethyl-1,4-cyclohexadiene in Organic Syntheses Article
- reduction of benzoic acid to 2,5-Cyclohexadiene-1-carboxylic acid in Organic Syntheses Article
References
[edit | edit source]- ↑ * A. J. Birch, J. Chem. Soc. 1944, 430.
<< Aromatics | Aromatic reactions | Ketones and aldehydes>>


