Organic Chemistry/Aromatics
<< Dienes | Aromatics | Aromatic reactions>>
After understanding the usefulness of unsaturated compound, or conjugated system, we hope to explore the unique structure of aromatic compounds, including why benzene should not be called 1,3,5-cyclohexatriene because it is more stable than a typical triene, and seemingly unreactive. Called "aromatic" initially because of its fragrance, aromaticity now refers to the stability of compounds that are considered aromatic, not only benzene. Any cyclic compound with 4n+2 pi electrons in the system is aromatic. The stability of aromatic compounds arises because all bonding orbitals are filled and low in energy.
History of Aromatics
[edit | edit source]Early in the 19th century, advances in equipment, technique and communications resulted in chemists discovering and experimenting with novel chemical compounds. In the course of their investigations they stumbled across a different kind of stable compound with the molecular formula of C6H6. Unable to visualize what such a compound might look like, the scientists invented all sorts of models for carbon-to-carbon bonding -- many of which were not entirely stable -- in order to fit what they had observed to what they expected the C6H6 compound to look like.
Benzene (which is the name that was given to the aromatic compound C6H6) is probably the most common and industrially important aromatic compound in wide use today. It was discovered in 1825 by Michael Faraday, and its commercial production from coal tar (and, later on, other natural sources) began in earnest about twenty-five years later. The structure of benzene emerged during the 1860s, the result of contributions from several chemists, most famously that of Kekulé.
Scientists of the time did not have the benefit of understanding that electrons are capable of delocalization, so that all carbon atoms could share the same π-bond electron configuration equally. Huckel was the first to apply the new theory of quantum mechanics to clearly separating σ and π electrons. He went on to develop a theory of π electron bonding for benzene, which was the first to explain the electronic origins of aromaticity.
Benzene Structure
[edit | edit source]Benzene is a hexagonal ring of six carbon atoms connected to each other through one p-orbital per carbon. Its chemical formula is C6H6, and its structure is a hexagonal ring of carbons sharing symmetrical bonds, with all six hydrogen atoms protruding outwards from the carbon ring, but in the same plane as the ring. The p-orbital system contains 6 electrons, and one way to distribute the electrons yields the following structure:
However, another resonance form of benzene is possible, where the single bonds of the first structure are replaced with double bonds, and the double bonds with single bonds. These two resonance forms are co-dominant in benzene. (Other forms, such as a structure with a π bond connecting opposite carbons, are possible but negligible.) Thus, each bond in benzene has been experimentally shown to be of equal length and strength, and each is accounted as approximately a "1.5" bond instead of either a single or double bond alone.
Electron density is shared between carbons, in effect yielding neither a single nor a double bond, but a sort of one-and-a-half bond between each of the six carbons. Benzene has a density of negative charge both above and below the plane formed by the ring structure. Although benzene is very stable and does not tend to react energetically with most substances, electrophilic compounds may be attracted to this localized electron density and such substances may form a bond with the aromatic benzene ring.
An electron delocalisation ring can be used to show in a single picture both dominant resonance forms of benzene:
Benzene Properties
[edit | edit source]Benzene is a colorless, flammable liquid with a sweet aroma and carcinogenic effects. The aromatic properties of benzene make it far different from other alkenes in many ways.
Benzene Reactions
[edit | edit source]- Main article: Aromatic reactions
Unlike alkenes, aromatic compounds such as benzene undergo substitution reactions instead of addition reactions. The most common reaction for benzene to undergo is electrophilic aromatic substitution (EAS), although in a few special cases, substituted benzenes can undergo nucleophilic aromatic substitution.
Benzene Health Effects
[edit | edit source]In the body, benzene is metabolized, and benzene exposure may have quite serious health effects. Breathing in very high levels of benzene can result in death, while somewhat lower (but still high) levels can cause drowsiness, dizziness, rapid heart rate, headaches, tremors, confusion, and unconsciousness. Eating or drinking foods containing high levels of benzene can cause vomiting, irritation of the stomach, dizziness, sleepiness, convulsions, rapid heart rate, and even death.
The major effect of benzene from chronic (long-term) exposure is to the blood. Benzene damages the bone marrow and can cause a decrease in red blood cells, leading to anemia. It can also cause excessive bleeding and depress the immune system, increasing the chance of infection. Some women who breathed high levels of benzene for many months had irregular menstrual periods and a decrease in the size of their ovaries. It is not known whether benzene exposure affects the developing fetus in pregnant women or fertility in men, however animal studies have shown low birth weights, delayed bone formation, and bone marrow damage when pregnant animals breathed benzene.
The US Department of Health and Human Services (DHHS) also classifies benzene as a human carcinogen. Long-term exposure to high levels of benzene in the air can cause leukemia, a potentially fatal cancer of the blood-forming organs. In particular, Acute Myeloid Leukemia (AML) may be caused by benzene.
Aromaticity
[edit | edit source]Aromaticity in organic chemistry does not refer to whether or not a molecule triggers a sensory response from olfactory organs (whether it "smells"), but rather refers to the arrangement of electron bonds in a cyclic molecule. Many molecules that have a strong odor (such as diatomic chlorine Cl2) are not aromatic in structure -- odor has little to do with chemical aromaticity. It was the case, however, that many of the earliest-known examples of aromatic compounds had distinctively pleasant smells. This property led to the term "aromatic" for this class of compounds, and hence the property of having enhanced stability due to delocalized electrons came to be called "aromaticity".
Definition
[edit | edit source]Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. It can also be considered a manifestation of cyclic delocalization and of resonance.
This is usually considered to be because electrons are free to cycle around circular arrangements of atoms, which are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly-seen model of aromatic rings was developed by Kekulé. The model for benzene consists of two resonance forms, which corresponds to the double and single bonds' switching positions. Benzene is a more stable molecule than would be expected of cyclohexatriene, which is a theoretical molecule.
Theory
[edit | edit source]By convention, the double-headed arrow indicates that two structures are simply hypothetical, since neither can be said to be an accurate representation of the actual compound. The actual molecule is best represented by a hybrid (average) of most likely structures, called resonance forms. A carbon-carbon double bond is shorter in length than a carbon-carbon single bond, but aromatic compounds are perfectly geometrical (that is, not lop-sided) because all the carbon-carbon bonds have the same length. The actual distance between atoms inside an aromatic molecule is intermediate between that of a single and that of a double bond.
A better representation than Lewis drawings of double and single bonds is that of the circular π bond (Armstrong's inner cycle), in which the electron density is evenly distributed through a π bond above and below the ring. This model more correctly represents the location of electron density within the aromatic molecule's overall structure. The single bonds are sigma (σ) bonds formed with electrons positioned "in line" between the carbon atoms' nuclei. Double bonds consist of one "in line" σ bond and another non-linearly arranged bond -- a π-bond. The π-bonds are formed from the overlap of atomic p-orbitals simultaneously above and below the plane of the ring formed by the "in line" σ-bonds.
Since they are out of the plane of the atoms, π orbitals can interact with each other freely, and thereby they become delocalized. This means that, instead of being tied to one particular atom of carbon, each electron can be shared by all the carbon atoms in an aromatic ring. Thus, there are not enough electrons to form double bonds on all the carbon atoms, but the "extra" electrons strengthen all of the bonds of the ring equally.
Characteristics
[edit | edit source]An aromatic compound contains a set of covalently-bound atoms with specific characteristics:
- The molecule has to be cyclic
- A delocalized conjugated pi system, most commonly an arrangement of alternating single and double bonds (can sometimes include triple bonds if the geometry of the molecule permits)
- Coplanar structure, with all the contributing atoms in the same plane
- A number of pi delocalized electrons that is even, but not a multiple of 4. (This is known as Hückel's (4n+2)Π rule, where,n= 0,1,2,3 and so on. Permissible numbers of π electrons include 2, 6, 10, 14, and so on)
- Special reactivity in organic reactions such as electrophilic aromatic substitution and nucleophilic aromatic substitution
Whereas benzene is aromatic (6 electrons, from 3 double bonds), cyclobutadiene is not, since the number of π delocalized electrons is 4, which is not satisfied by any n integer value. The cyclobutadienide (2−) ion, however, is aromatic (6 electrons). An atom in an aromatic system can have other electrons that are not part of the system, and are therefore ignored for the 4n + 2 rule. In furan, the oxygen atom is sp2 hybridized. One lone pair is in the π system and the other in the plane of the ring (analogous to C-H bond on the other positions). There are 6 π electrons, so furan is aromatic.
Aromatic molecules typically display enhanced chemical stability, compared to similar non-aromatic molecules. The circulating (that is, delocalized) π electrons in an aromatic molecule generate significant local magnetic fields that can be detected by NMR techniques. NMR experiments show that protons on the aromatic ring are shifted substantially further down-field than those on aliphatic carbons. Planar monocyclic molecules containing 4n π electrons are called anti-aromatic and are, in general, destabilized. Molecules that could be anti-aromatic will tend to alter their electronic or conformational structure to avoid this situation, thereby becoming merely non-aromatic.
Aromatic molecules are able to interact with each other in so-called π-π stacking: the π systems form two parallel rings overlap in a "face-to-face" orientation. Aromatic molecules are also able to interact with each other in an "edge-to-face" orientation: the slight positive charge of the substituents on the ring atoms of one molecule are attracted to the slight negative charge of the aromatic system on another molecule.
Monosubstituted Benzenes
[edit | edit source]Benzene is a very important basic structure which is useful for analysis and synthesis in most aspects of organic chemistry. The benzene ring itself is not the most interesting or useful feature of the molecule; which substitutents and where they are placed on the ring can be considered the most critical aspect of benzene chemistry in general.
Effects of Different Substituents
[edit | edit source]Depending on the type of substituent, atoms or groups of atoms may serve to make the benzene ring either more reactive or less reactive. If the atom or group makes the ring more reactive, it is called activating, and if less, then it is called deactivating.
Generally, the terms activating and deactivating are in terms of the reactions that fall into the category of Electrophilic Aromatic Substitution (EAS). These are the most common forms of reactions with aromatic rings. Aromatic rings can undergo other types of reactions, however, and in the case of Nucleophilic Aromatic Substitution, the activating and deactivating nature of substituents on the rings is reversed. In EAS, a hydroxyl groups is strongly activating, but in Nucleophilic Aromatic Substitution, a hydroxyl group is strongly deactivating. But since EAS is the most common reaction with aromatic rings, when discussing activation and deactivation, it's normally done in terms of the EAS.
In addition to activating or deactivating, all groups and/or substituent atoms on a benzene ring are directing. An atom or group may encourage additional atoms or groups to add or not to add to certain other carbons in relation to the carbon connected to the directing group. This concept will be further discussed in the next chapter, but when memorizing the groups below it is helpful to also memorize whether it is O (ortho), M (meta) or P (para)-directing.
Another factor that heavily influences direction, however, is steric hindrance. If, for example, you have a tert-butyl substituent on the ring, despite the fact that it is ortho/para directing, the ortho positions will be largely blocked by the tert-butyl group and thus nearly all the product would be para.
Activating Substituents
[edit | edit source]Activating substituents make benzene either slightly more reactive or very much more reactive, depending on the group or atom in question. In general, if one of the major heteroatoms (nitrogen or oxygen) is directly attached to the carbon ring then the result is probably activation. This is merely a rule of thumb, and many exceptions exist, so it is best to memorize the groups listed below instead of counting on a quick and dirty rule of thumb.
| Group | Strength | Directing |
| -NH2, -NHR, -NRR | very strong | ortho/para |
| -OH, -O- | very strong | ortho/para |
| -NHCOCH3, -NHCOR | strong | ortho/para |
| -OCH3, -OR | strong | ortho/para |
| -CH3, -C2H5, -R | weak | ortho/para |
| -C6H5 | very weak | ortho/para |
Deactivating Substituents
[edit | edit source]A deactivating group is a functional group attached to a benzene molecule that removes electron density from the benzene ring, making electrophilic aromatic substitution reactions slower and more difficult than they would be on benzene alone. As discussed above for activating groups, deactivating groups may also determine the positions (relative to themselves) on the benzene ring where substitutions take place, so each deactivating group is listed below along with its directing characteristic.
| Group | Strength | Directing |
| -NR3+ | very strong | meta |
| -NO2 | very strong | meta |
| -CF3, CCl3 | very strong | meta |
| -CN | strong | meta |
| -SO3H | strong | meta |
| -CO2H, -CO2R | strong | meta |
| -COH, -COR | strong | meta |
| -F | weak | ortho/para |
| -Cl | weak | ortho/para |
| -Br | weak | ortho/para |
Activation vs. Deactivation and ortho/para vs. meta directing
[edit | edit source]So why are some substituents activating or deactivating? Why are some meta directing and others ortho/para directing? From the above tables, it seems pretty clear there's a relationship.
There are primarily two effects that substituents impart on the ring that affect these features:
- Resonance effects
- Inductive effects
Resonance Effects
[edit | edit source]Let's first look at resonance effects. Resonance effects are the ability or inability of a substituent to provide electrons to the ring and enhance its resonance stability. To see this, we must first get a basic understanding of the mechanism of Electrophilic Aromatic Subsitution. We'll discuss EAS in more detail in the next section, but some basics are called for here.

As you can see in the image above, the electrophile is attacked by pi electrons in the ring. The same carbon is now bonded to both the hydrogen that was bonded to it and the electrophile. This in turn creates a carbocation on the adjacent carbon, making the ring non-aromatic. But aromatic rings like to remain aromatic. The nucleophile which was previously bonded to the electrophile now attacks the hydrogen, abstracting it from the ring and allowing the pi-bond to re-form and returning the ring to its aromatic nature.
As we've seen before in some other reactions, when a carbocation is created as an intermediate, stability of that carbocation is crucial to the reaction. This is the case in Electrophilic Aromatic Subsitution as well.
So what is the effect of substituents on the ring?
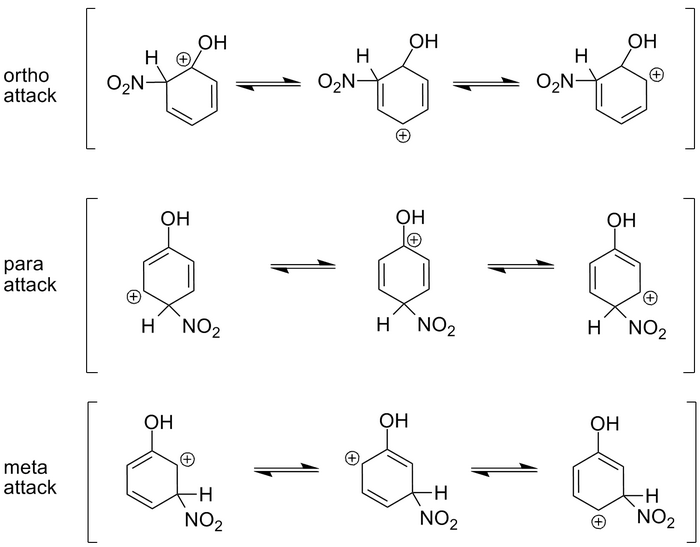
Let's look at the situation above. In this case we have Phenol, a benzene ring with an -OH (hydroxyl) group attached. When we nitrate the ring with nitric acid in sulfuric acid (a reaction we'll discuss in the next section), a nitro group is attached to the benzene ring.
There are 3 possible places for the nitro group to attach: An ortho, meta, or para position. To understand the stability of the carbocation, we need to look at the resonance structures for a given attack and see what the results are.
The first resonance structure of the ortho attack results in a positive charge on the carbon with the hydroxyl group. This happens to be the most stable of the 3 resonance structures for an ortho attack because the two negative electron pairs in the oxygen act to stabilize the positive charge on the carbon. The other two resonance forms leave a carbon with a hydrogen attached, to hold the positive charge. Hydrogen can do nothing to stabilize the charge and thus, these are less stable forms.
In the para attack situation, notice that the second resonance form also puts a positive charge on the carbon with the hydroxyl group. This provides for stability just as it does in the case of an ortho attack and thus, the middle resonance form is very stable.
Finally, in the meta attack situation, all of the resonance forms result in a positive charge on a carbon with only a hydrogen attached. None of these is stable, and thus, meta attack with a hydroxyl group attached, is a very small percentage of the product.
So the electron pairs in the oxygen act to stabilize the ortho and para attacks.
Inductive Effects
[edit | edit source]Now let's look at the inductive effects of deactivating substituents. Let's imagine that, instead of a hydroxyl group, we instead have a carbonyl group attached to the ring in its place. When a carbonyl is attached, the ring is bonded to a carbon which in turn, is double-bonded to an oxygen, the double-bonded oxygen is withdrawing electrons and this inductive effect is felt on the ring, strongly deactivating its pi-bond nature and putting a positive dipole on the carbon. Looking at the resonance structures, this carbon, which already has some positive nature is now given the added resonance of a positive charge, in the case of ortho and para attacks. Positive plus positive equals more positive and thus, less stable. There's no negative charge or negative electron pair to stabilize this positive charge.
So in this case, not only is the entire ring less activated, but the ortho and para attacks result in much more unstable carbocation resonance forms. Hence, meta is the preferred position, but the overall reaction is less active than plain benzene.
Halides as the Exception
[edit | edit source]Notice that in the list of activating vs. deactivating substituents, the activating ones are all ortho/para directing. In the deactivating substituents, all but the halides, are meta directing. Why are halides an exception?
Because halides are more electronegative than carbon, they induce a positive dipole on the attached carbon and a negative dipole on their own atom(inductive effect), and in accordance to the previous logic of activating/deactivating substituents, deactivate the ring. However, halides also possess lone pair electrons in their outer shell to share with the ring, allowing the resonance structures with favored ortho/para attacks versus meta attacks due to their poor resonance forms. In essence, although halides do deactivate the ring to some extent, they provide major resonance contributors due to the availability of their lone pairs. Resonance structures usually trump the inductive effect.
Detailed Effects of Substituents
[edit | edit source]We've discussed some generalities about the effects of substituents and even some specifics about certain ones, but let's look more closely at the substituents and try to understand the details of what makes them activating vs. deactivating.
-NH2, -NHR, and -NRR are all very strongly activating. Though nitrogen is more electronegative than carbon, its ability to share a pair of electrons greatly outweighs its electron withdrawing effect.
-OH and -O- is similar in that it is even more electronegative than nitrogen, but it has two pairs of electrons to share, which also greatly outweighs its electron withdrawing effect.
-NHCOCH3 and -NHCOR are also strongly activating, but the inductive effect of the double-bonded oxygen acts to make the nitrogen more electron withdrawing, so they're not quite as activating as the other -N substituents above.
-OCH3 and -OR are also still strongly activating, but less so, because the electron density is shared on both sides of the oxygen.
-CH3 and -R in general provide some electron density sharing, but not nearly as much as a pair of electrons. Thus their effect is only weakly felt.
For deactivating groups we have:
-NO2, or nitro and -NR3+. The nitro group is very strongly deactivating because of its resonance structure. The nitro group has two resonance forms: O=N+-O- and O--N+=O. Both of these forms leave a full positive charge on the nitrogen making it completely unable to help stabilize the positive carbocation intermediate. The same applies to -NR3+.
-CF3 and -CCl3 both have an inductive electronegative effect of 3 halides, but with no electrons to share with the ring, leaving them also very strongly deactivating.
-CN has a triple bond between the carbon and nitrogen with a resonance form of a double bond between the carbon and nitrogen and a positive charge on the carbon, meaning that between the electronegativity of the nitrogen and positively charged carbon in the resonance form, it destabilizes the carbocation and offer no electrons to the ring.
-SO3, -COR, -CO2R - all of these have electronegative oxygens giving the carbon a positive partial charge and providing no electrons for stability on the ring.
-F, -Cl, -Br, all have a similar effect. They are electronegative and deactivate the ring, but have electrons to share that, to some degree, makes up for it, allowing the ortho/para direction. But to understand their effects better, you need to look at them in terms of their placement on the periodic chart. Florine is the most electronegative element and it's very small and thus very close to the carbon it's bonded to. This gives its electromagnetic influence a stronger deactivating character. Chlorine is less electronegative, but it's also larger and thus further away from the carbon, making it harder for it to share its electrons. And so on.
Polysubstituted Benzenes
[edit | edit source]Unsubstituted benzene is seldom encountered in nature or in the laboratory, and you will find in your studies that most often benzene rings are found as parts of other, more complicated molecules. In order for benzene to react in most situations, it gains or loses some functionality dependent on which functional groups are attached. Although the simplest case is to work with benzene that has only one functional group, it is also essential to understand the interactions and competitions between multiple functional groups attached to the same benzene ring.
When there is more than one substituent present on a benzene ring the spatial relationship between groups becomes important, which is why the arene substitution patterns ortho, meta and para were devised. For example three isomers exist for the molecule cresol because the methyl group and the hydroxyl group can be placed either next to each other (ortho), one position removed from each other (meta) or two positions removed from each other (para). Where each group attaches is most often a function of which order they were attached in, due to the activating/deactivating and directing activities of previously attached groups.
Competition Between Functional Groups
[edit | edit source]When a ring has more than one functional group, the effects of the groups are combined and their total effect must be taken into account. In general, effects are summed. For example, toluene (methylbenzene) is weakly activated. But p-nitrotoluene has both a methyl group and a nitro group. The methyl group is weakly activating and the nitro is pretty strongly deactivating, so overall, the group is very deactivated. In terms of direction, however, both substitutents agree on the direction. The methyl group is ortho/para directing. The nitro group occupies the para position, so the methyl will now want just ortho direction. The nitro group is meta directing. The positions meta to the nitro are also ortho to the methyl, so this works out and further substituents will be almost entirely in the positions ortho to the methyl group.
If two functional groups disagree on direction, the more activating group is the one that controls direction. That is, if you had m-nitrotoluene, most of your product would tend to be ortho/para to the toluene and not meta to the nitro, despite the nitro having a stronger influence on overall activation.
Naming Conventions
[edit | edit source]When a benzene ring has more than one substituent group attached, the location of all of the groups not directly attached to carbon number one must be explicitly declared. This is done by listing the number of the carbon atom where the group is attached, followed by a hyphen and the group's name. The carbon atoms of the benzene ring should be numbered in order of previously established precedence, i.e., a bromine would take precedence over a nitro group, which itself would take precedence over an alcohol or alkane group. The names of the groups should be listed in alphabetical order, i.e. "2-methyl-5-nitrobenzaldehyde."
<< Dienes | Aromatics | Aromatic reactions>>