Structural Biochemistry/Nucleic Acid/DNA/DNA structure/Telomeres
Telomeres (from the Greek telos, "an end") are long stretches of repeating non-coding DNA sequences at the ends of the DNA strand. They protect the ends of DNA and prevent DNA strands from shortening or attaching to other molecules by masking the chromosome. Russian Alexei Olonikov was the first to postulate the problem of chromosomes replicating at the tip.[1] He theorized that in every subsequent replication bits of the DNA would be lost until a critical limit had been reached, thereupon cell division would cease.
Telomerase[edit | edit source]

Telomerase is an enzyme that creates the Telomeres. Telomerase adds specific repeating sequences ("TTAGGG" in all vertebrates) to the ends of four DNA strands.
[2]
The telomerase enzyme has an RNA template that partially attaches to the shortened end of the DNA strand. New nucleic acids then attach to the template, extending the DNA strand. Once the telomerase leaves, the double stranded DNA is completed with the DNA polymerase. Telomerase was discovered in 1985 by Carol W. Greider and Elizabeth Blackburn. For this discovery, they were awarded the 2009 Nobel Prize in Physiology or Medicine along with Jack W. Szostak.[3]
Szostak and Blackburn first discovered telomeres in ciliates. They chose ciliates because at one stage of their life cycle, they make a million new telomeres. The model created includes a telomere-dedicated DNA polymerase, which adds telomeric repeats onto chromosome ends. Therefore, telomeres are represented as a motif in DNA sequences.
Telomerase's presence in humans is somewhat strange. It is located in the nucleus which is unsurprising because that is where DNA replication takes place. However, Telomerase activity is not present in all cells. It was found to be almost absent in the majority of normal adult tissues, including cardiac and skeletal muscle, lung, liver, and kidney. Because of this curious lack of telomerase activity, a theory arose connecting telomere length to aging and cell senescence. According to this theory, human somatic cells are born with a full number of telomeric repeats, but the telomerase enzyme is not present in some tissues. The cells of those tissues would lose about 50 to 100 nucleotides from each chromosome end each time they underwent replication and division. Eventually, the telomeres would cease to exist and the chromosomes themselves would start losing nucleotides, carrying genetic defects into their next division so that neither daughter cell would be viable. Thus after a certain number of divisions a cell will not have enough nucleotides and die.[4]
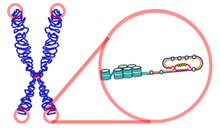
The function of Telomerase is to allow for short replacements of Telomeres which are gradually lost during cell division.[5] In normal conditions without Telomerase, a cell would divide until it would hit a critical point known as the Hayflick limit.[6] In the presence of Telomerase, however, the cell has the ability to replace lost DNA and divide without limit. But this continuous growth comes with a consequence as this growth may lead eventually to cancerous cells.
While the details are not fully known, it would seem that that shortened Telomeres play a role in aging due to the erosion of the DNA over time. The questions arises whether or not Telomerase has the ability to greatly extend the lifespan of a human due to its importance in the maintenance of the Telomeres.[7]Dr. Michael Fossel, a professor of clinical medicine at Michigan State University, has expressed his views on Telomerase as a viable treatment for cell senescence.
However, several experiments have raised doubts on the ability of Telomerase as an effective anti-aging treatment. An experiment was done with mice having higher levels of Telomerase and it was discovered that they also had a higher rate of cancer which therefore led to a shorter lifespan. In addition, Telomerase favors tumorogenesis.[8] Telomerase fosters cancer development by allowing uncontrolled cell growth which eventually proliferates into tumors. In fact, Telomerase activity has been observed in approximately 90% of all human tumors which suggests that the uncontrolled growth of a cell as conveyed by Telomerase has a key role in cancer.
In addition to using Telomerase as an anti-aging treatment, Telomerase has potential as a drug target against cancer.[9] Since it is necessary for the immortality of many cancer cell types, it is believed that if a drug is able to deactivate Telomerase activity in a cell, Telomeres would shorten, mutations would happen, cell stability would decrease and cancer would be, in essence, effectively treated. Experimental drugs have been tested in mouse models and some drugs have moved onto clinical testing.
Cancer Biology[edit | edit source]
The significance of studying telomeres can be found in telomerase, which rebuilds the telomere so that the cells can keep dividing. The telomerase, however, eventually shortens the telomere, causing the cell to die. In the case of cancer cells, this enzyme builds telomeres long past the cell's average lifetime. These cells then are called to be "immortaled", since they can divide endlessly. This results in a tumor. Many researchers believe that telomere maintenance activity is characterized in most human cancer cells. Though the mechanism by which such phenomena happen has not been well understood, the discovery may reveal key elements of telomere function. Telomerase, on the other hand, is the natural enzyme used for telomere repair, highly abundant in stem cells, germ cells, hair follicles, and most cancers cells, but its expression is low or in some cases absent in somatic cells. Telomerase functions by adding bases to the ends of the telomeres. Cells with sufficient telomerase activity are considered immortal in the sense that they can divide past the Hayflick limit without entering senescence or apoptosis. For this reason, telomerase is viewed as a potential target for anti-cancer drugs such as telomestatin.
2009 Nobel Prize[edit | edit source]
The Nobel Prize 2009 in Physiology and Medicine was awarded to three scientists who have discovered how the chromosomes can be copied in a complete way during cell divisions and how they are protected against degradation. By showing that the ends of the chromosomes, telomeres, and their enzyme, telomerase, are significant in protecting the chromosomes from degradation, they identified telomerase and explained how the telomeres protect the ends of the chromosomes and built by telomerase. On the other hand, if the telomeres become shortened, cells can duplicate damaged as cancer cells. If telomerase is well maintained, conversely, telomere length is maintained and the cell does not become cancerous. In the case of cancer cells, telomerase allows the cell to divide without any limit. Certain genetic disease are caused by a defective telomerase. This discovery can thus be used to stimulate the development of new therapeutic strategies. Understanding such fundamental mechanism is an important first step toward opening new doors for cures for cancer and other related diseases, as well as anti-aging.
Hayflick Limit[edit | edit source]
The Hayflick limit is the number of times a normal cell may divide until it reaches a critical limit and stops dividing based on the idea that Telomeres reach a critical length.[10] This limit was discovered by Leonard Hayflick in the 1960s who demonstrated that the cells in a normal fetus divided around 40 to 60 times before entering into cell senescence. Due to repeated mitosis, the Telomere shortening occurred which inhibited cell division which is analogous to aging. The discovery of this limit, a pillar of Biology, refuted the early contention by Alexis Carrel who, along with the majority of scientists during that time period, believed cells were "immortal".
Role of Telomere[edit | edit source]
Telomeres account for the lost bits of DNA at the ends of chromosomes during DNA replication. Since DNA polymerase moves along the template strand in the 5'--> 3' direction, some of the 5' end of the template strand will not be replicated. This results in the incomplete ends as shown in the diagram below. However, telomeres are usually very long, ranging from 400 to 600 base pairs in yeast to many kilobases in humans. They are made of six to eight base pair long repeats which are usually rich with guanine bases. With long stretches of telomeres at the ends of DNA strands, the incomplete strands of DNA will still contain the genetic code.

The shortening of telomeres in humans induces cell senescence in humans. This mechanism appears to cause the formation of cancerous cells. Telomere length has been theorized in recent publications to account for the aging in humans. Since cells replicate identically, there must be a reason why cells within a body lose function and viability with time. Telomeres may have some influence over the aging process since every consequent DNA replication results in the shortening of telomeres. Two aspects to this question are: (i) whether telomere length, as measured in specific cell populations in the body, correlates with longevity or disease; and (ii) whether telomere shortening in any cell population causes functional impairment of that cell population. However, some may argue telomeres do not correlate to longevity as mice contain long strands of telomeres, but contrarily live much shorter lives than humans who do not have as long telomeres as do mice. And some may argue that telomere length does correlate to longevity as it determines the number of times that a cell can divide before it dies or reaches senescence.
Recent Publications[edit | edit source]
Recently it has been found that telomerase activity is inversely related to length of the telomeres. In other words, telomere elongation happens more often on short telomeres rather than long ones. The research showed a deficiency in telomerase activity in telomeres greater than 125 base pairs,and there was 2 to 3 times more telomerase activity in telomeres shorter than 125 base pairs. This preferential elongation has been demonstrated in yeast and mice, and now human somatic cells. Kinetic data indicates that elongation in yeast cells in a single event in which elongates the telomeres to a certain length, whereas in human cells the elongation seems to be a gradual process. The researchers showed that telomerase adds a regulated length of telomere in each cell division. The researchers showed that human cells expressed telomerase, however long telomeres were maintained and not elongated where as the cells with shorter telomeres elongated, which goes to show that telomeres can not be infinitely extended.[11]
Another interesting paper was focused on the role of DNA damage response (DDR) proteins in the role of telomere maintenance. The review says that early stage DNA repair proteins have a significant role in telomere maintenance where as late stage proteins usually do not take part in telomere repair. The interplay with these proteins and the proteins that cap the telomeres to protect the telomeres is very important too. Many of stronger DDR proteins inhibit cell replication, because of this fact, it would be harmful to the organism for these proteins to be a part of telomere repair. These protein caps on the telomeres inhibit full DNA damage response which keeps the stronger protein from "repairing" the telomere ends. It still isn't clear why some of the DDR proteins participate in telomere maintenance and others do not, but it is clear that the cellular process in repairing a DNA break and repairing telomeres are two different process, with the former halting cellular division.[12]
References[edit | edit source]
- ↑ "Telomeres, telomerase, and aging: Origin of the theory". Alexey M. OlovnikovE-mail The Corresponding Author. 1999. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ "Repeat Expansion–Detection Analysis of Telomeric Uninterrupted (TTAGGG)n Arrays". [1]. 2007. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ "The Nobel Prize in Physiology or Medicine 2009". [2]. 2009. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ "What are telomeres and telomerase?". [3]. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ "Telomerase: regulation, function and transformation". [4]. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ "Hayflick Limit Theory". [5]. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ "Extension of Life-Span by Introduction of Telomerase into Normal Human Cells". [6]. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ "Anti-Aging Medicine". João Pedro de Magalhães. 2008. Retrieved 2009-11-05.
{{cite web}}: External link in|publisher= - ↑ Foreman, Judy. "Telomerase - a Promising Cancer Drug Stuck in Patent Hell?". myhealthsense.com. Retrieved 2009-11-05.
- ↑ "Cellular Senescence". João Pedro de Magalhães. 2008. Retrieved 2009-11-17.
{{cite web}}: External link in|publisher= - ↑ Britt-Compton, Bethan; Capper, Rebecca; Rowson, Jan; Baird, Duncan M. (2009). FEBS Letters (583): 3076–3080.
{{cite journal}}: Missing or empty|title=(help) - ↑ Lyndall, David (2009). The EMBO Journal (28): 2174–2187.
{{cite journal}}: Missing or empty|title=(help)