Structural Biochemistry/Endergonic reaction
General information
[edit | edit source]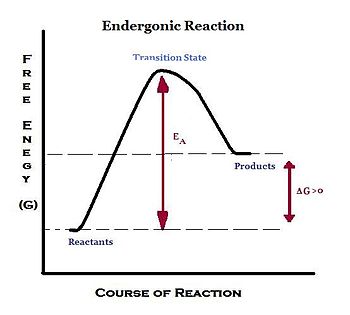
An endergonic reaction refers to a chemical reaction in which energy is being used in the overall reaction, making the reaction non-spontaneous and thermodynamiacally unfavorable. Energy is being absorbed as the reaction proceeds, and there is a net loss of energy in the surrounding system. Due to this consumption of energy, standard change in Gibbs free energy (ΔG) is a positive value under constant pressure and temperature: ΔG° > 0.
The magnitude of ΔG also represents the quantity of energy required to drive the reaction. If a chemical process is exergonic in one direction, then the reverse process must be endergonic. Plants get the required energy to make sugar from the environment by capturing light and converting it into chemical energy that can be used for other processes.
Some examples of endergonic reactions are muscle contractions and protein synthesis.
The equilibrium constant of endergonic reaction where ΔG° > 0 is less than 1: K < 1.
Endergonic reactions require an input of energy, usually larger than those of non spontaneous exergonic reactions, from an outside source to disturb the chemical equilibrium to cause changes, such as bond formation. This input of energy is called the activation energy. In certain reactions, a catalyst is available to speed up endergonic reactions. A catalyst can lower the activation energy barrier for the reaction. Thus, it speeds up the reaction process. The energy for an endergonic reaction is obtained by coupling the reaction with an exergonic reaction.
A familiar example of coupling exergonic reactions to endergonic reactions to promote spontaneity comes from ATP. ATP powers cellular work by coupling exergonic reactions to endergonic reactions. It is responsible for mediating most energy coupling in cells, and in most cases, acts as the immediate source of energy that powers cellular work. The synthesis of the amino acid glutamine from glutamic acid and ammonia is naturally endergonic and non spontaneous with a ΔG value of +3.4 kcal/mol but coupling this reaction with the exergonic process of ATP hydrolysis, -7.3 kcal/mol, will drive the reaction forward, making it spontaneous. In the same sense, a exergonic reaction must be coupled with the formation of ATP from ADP in order to make the reaction spontaneous and in most cases, cellular respiration provides the energy for the endergonic process of making ATP and plants use light energy, instead, to produce ATP.
An endergonic reaction can simply be understood by studying the following situation.
In a chemical reaction, the reactants make products and an equilibrium is reached. An endergonic reaction is one where more products are made from the equilibrium amount by disturbing the equilibrium with a form of energy. For example, heat will be absorbed into the system and the equilibrium will shift to the right (towards product side). Consequently, more products will be formed.
Summary
[edit | edit source]An endergonic reaction:
- The free energy of initial state < free energy of final state
- Energy needs to be put into the system in order to go from the initial start to the final state
- +ΔG
References
[edit | edit source]Zumdahl, Chemistry Seventh Edition
Neil A. Campbell, Jan B Reece. Biology Seventh Edition, 2005 Pearson Education, Inc.
http://academic.brooklyn.cuny.edu/biology/bio4fv/page/exergon.htm
