Structural Biochemistry/WD40 Domains
WD40 Domains
[edit | edit source]They are the most abundant domain proteins in eukaryotic genomes. As scaffolds, WD40 domain proteins are involved in a variety of cellular process such as signal transduction, cell division, cytoskeleton assembly, chemotaxis and RNA processing. Most distinctive feature of the WD domains is that they mediate diverse interactions between protein and another protein.

Structure
[edit | edit source]WD40 domains have highly symmetrical structure, which supports more rapid and convenient folding. Also, the domains are characterized by the structure of propellers with seven blades. WD40 propellers have three distinctive surfaces, which are top, the bottom, and the circumference, and they take part in various interactions. The top region is the part of the structure where the loops lie, and the loops connect D and A strands of the WD repeats.
WD40-peptide interactions
[edit | edit source]Most protein-protein and protein-peptide interactions happen at the specific site of the central channel of the β-propeller. The site is called supersite and this site is and at this site, most of interaction partners bind. Most interactions occur on the top surface of the propeller
Since the core of the central channel is not accessible for interactions and N- or C-terminal of the β-propeller pack against the entry sties of the central channel, majority of interactions happen in WD40 domains. For example, one of the interactions that WD40 domains participate in is the interaction between globular proteins and those involving short peptides/linear motifs.
Peptide-protein interactions
The interaction between WD40 and peptide is important for the assembly of dynamic multi-protein complexes. When the interaction happens, many different peptide motifs bind to different sites of the propeller, but most peptides bind on the top of β propeller close to the central channel. The binding site variability gives WD40 domains the characteristics as ideal platforms for assembling different kinds of proteins and helping the formation of transient complexes.
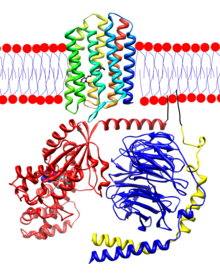
Transducin
[edit | edit source]Transducin, which is responsible for the signal transmission from rhodopsin to cyclic guanosine monophosphate phosphodiesterase, consists of three subunits : α-, β-, and γ- subunits
α-subunit
It consists of a GTPase domain. A GTPase domain interacts with the α-helical domain , which does not interact with WD40 domain and an N-terminal helix, which contacts the side of the propeller
β-subunit
It forms a stable heterodimer along with γ-subunit
γ-subunit
It consists of two helices. The first helix with an N-terminal helix precedes the β –subunit propeller, and the second helix contacts loops on the bottom of the propeller. γ-subunit controls the expression level of the entire transducin heterotrimer, which plays a major role for normal transducin localization.
References
[edit | edit source]- Stirnimann CU, Petsalaki E, Russell RB, Müller CW. "WD40 proteins propel cellular networks" Trends Biochem Sci. 2010 Oct;35(10):565-74. Epub 2010 May 5. Review
- Xu C, Min J. "Structure and function of WD40 domain proteins." Structural Genomics Consortium, University of Toronto, 101 College St., Toronto, Ontario, Canada
Sources
http://commons.wikimedia.org/wiki/File:Rhodopsin-transducin.png
http://commons.wikimedia.org/wiki/File:1erj_7bladed_beta_propeller.png
