Structural Biochemistry/Proto-oncogene
Proto-oncogene is a normal gene that is responsible for cell growth, cell differentiation, cell division, and apoptosis . However, these types of genes have the potential to become an oncogene, a cancer-causing gene.
If a cell can no longer control growth, death, and division, there can be huge health problems. Studying why and how to prevent proto-oncogenes from changing to oncogenes has been gaining more and more attention.

Causes
[edit | edit source]Mutations
[edit | edit source]Mutations in proto-oncogene can cause it to turn into an oncogene.
- Point-mutation: deletion or insertion
- Gene amplification event.
- Chromosomal translocation.
- Mutations in microRNA (miRNA): microRNAs regulate genes by down-regulating them, but mutations in miRNA can prevent them from suppressing the proper genes.
Effects
[edit | edit source]Just like any other cancer cells, oncogenes can cause many uncontrollable events.
- Loss of regulation
- Increase in enzyme activity
- Increase in protein concentration
Treatment
[edit | edit source]
Cancer cells go through many changes that are difficult to target individually. Scientists believe that cancerous cells depend on a certain oncogenes more than the others when it comes to cellular growth and replication. This characteristics is called oncogene addiction. If scientists can determine this specific gene, they can treat the cancer by blocking this gene.
Suppressing oncogenes to treat cancer
[edit | edit source]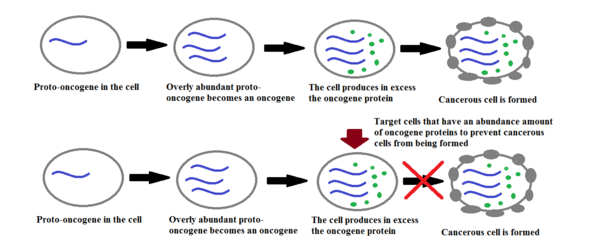
If scientists cannot target a certain gene and prevent it from causing cancer, then why not turn to a different method of treatment, such as suppressing that cancerous oncogene. When certain cells have oncogenes that are amplified, or they are present in excess, they produce large amounts of their proteins which can initiate the formation of cancerous cells and ultimately lead to tumors. In breast cancer for example, the proto-oncogene HER2/neu is present in normal tissue cells but it turns into an oncogene when the HER2/neu gene is present in large excess and it produces in abundance its respective HER2/neu protein in a cell. Scientists have concluded that, in breast cancer patients for example, cancerous cells that have a high amount of HER2/neu protein did not respond very well to medical treatments of chemotherapy as to those patients who did not have the HER2/neu protein in large excess in their cells. In order to help patients combat their cancer, new drugs have been made available to target the HER2/neu protein and thus reducing the growth of the cancerous cells and ultimately giving the patients a better chance of overcoming the cancer. Two drugs that are used to target the HER2/neu protein abundant cells are Herceptin® and Tykerb®, more drugs are currently undergoing clinical testing so that doctors can have a larger arsenal of drugs to help patients overcome cancer. In conclusion, by targeting cells that produce too much of the HER2/neu protein, which become cancerous, slows the progress of the cancer cells forming and thus making the cancer more easily treatable.[1]
Restoring tumor suppressant genes
[edit | edit source]An alternative way to treat cancer on a genetic level is to restore tumor suppressor genes that are not functioning as they should be. This method is more challenging for scientists because they have not been able to develop a successful way to do this. One large problem they face to solving the restoration of suppressant genes is a means to take the cancer cells and insert new DNA to make them function properly. The complexity of cancer cells and their potential multiple mutations only makes it even more so difficult because now there is more than one gene that needs to be replaced. One attempt that was carried out with the mutated gene TP53 where the scientists used a virus, that they had put the non-mutated TP53 gene into, to try to make it insert the normal gene into the cancerous cells. However, this was only successful when carried out in laboratory experiments and not successful in trials with patients. [1]
Reference
[edit | edit source][1] “Oncogenes, Tumor Suppressor Genes, and Cancer”. American Cancer Society, 27 Dec. 2011. Web. 20 Nov. 2012.