Structural Biochemistry/Protein function/Antibodies
An antibody is a protein that is synthesized by an animal in response to the presence of a foreign substance in our body, called an antigen. They play a great role in the immune system, and are usually found in blood and other bodily fluids. Antibodies are created by white blood cells, or more specifically, B cells. There are five isotypes of antibodies that each play self-defense role to fight off foreign objects in our body. Antibodies are created in response to antigens that include, but are not limited to, foreign proteins, polysaccharides, and nucleic acids. The antibody recognizes a small portion of the antigen called the antigenic determinant or epitope. Each antibody recognizes and binds to a specific antigen in a lock and key type model. Given the sheer amount of antigens present, there are an equally diverse selection of antibodies.
Structure
[edit | edit source]Antibodies are gamma globulin proteins that have sugar groups attached to amino acid chains. They can be classified as glycoproteins. The most basic form is the immunoglobulin monomer, which has only one immunoglobulin unit. Antibodies can also appear in dimeric forms with two immunoglobulin units, tetrameric form with four immunoglobulin units, or even pentameric form with five immunoglobulin units.
Immunoglobulin G, the most common type of antibody, consists of 4 chains. There are 2 light chains and 2 heavy chains. The two heavy chains are bound together by a disulfide bond (S-S), and the two light chains are bound to the heavy chain by disulfide bonds. Together, they roughly form a Y shape.
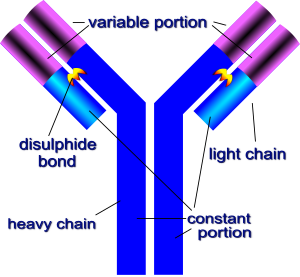
There are two sites that recognize and bind to antigens located at the top of the Y shaped immunoglobulin.Immunoglobulin G (IgG) all have the same general structure only varying at the antigen binding site. This region is called the variable region (V) and is composed of hypervariable loops. These hypervariable loops give great versatility to the antigen binding site allowing it to bind to multitudes of different antigens. The variable regions (V), which make up the two identical antigen-binding sties, are different in each specific type of antibody, giving these sites specific shapes that fit certain antigenic epitopes. The remainder of the molecule consists of light and heavy chain constant regions (C) where these amino acid sequences vary little form antibody to antibody.
Antibodies obtain their diversity through two processes. The first stage is called somatic or V(D)J, which stand for variable, diverse, and joining regions, recombination. Within each of the three regions are located several sets of genes. During cell maturation, the B cell will splice out the DNA of all but one of the genes from each region and combine the three remaining genes together to form one VDJ segment. This segment, along with a constant region gene, forms the basis for subsequent antibody production. It is estimated that given the number of variants in each of the three regions, approximately 10,000-20,000 unique antibodies are producible.
The second stage of recombination occurs after the B cell is activated by an antigen. When an antigen binds to the B cell, the B cell will begin to reproduce rapidly. During this division process, the variable regions of the gene will undergo rapid mutation called somatic hypermutation. This hypermutation serves to fine tune the antibody binding to the antigen. Cells that have a stronger affinity for the antigen will be given a stronger signal to multiply, leading to a gradual selection of antibodies that bind to the antigen the strongest.
In IgG, the heavy chain has four subunits, CH3, CH2, CH1 (the constant portions) and VH (the variable portion). The light chain has two subunits, CL and VL. The two CH3 units are joined directly, while the CH2 units are separated by oligosaccharides. The CH1 is located past the "hinge" of the heavy chain and is joined to the CL unit by a disulfide bond.
Folding
[edit | edit source]Because antibodies can be produced with thousands of variations, the chance of producing an antibody that cannot fold properly or will otherwise not function properly is high. Folding is therefore a very important step in antibody production, and antibody production is highly dependent on the "quality control" mechanisms of the endoplasmic reticulum (ER). Heavy chains and light chains are synthesized separately and translocated into the ER during the translation process; they begin folding before translation is even complete.
The Ig Fold
[edit | edit source]The Ig fold is a highly conserved protein topology, with such a broad presence in nature that it gives the name to the Ig superfamily (IgSF), which consists of proteins that contain an Ig fold. The Ig fold consists of two antiparallel β-sheets, containing 7 to 9 β-strands in total, which form a sandwich-like structure. Typically, the Ig fold is stabilized by internal disulfide bridges that connect two of the β-strands and run perpendicular to the sheets themselves. The two variable regions and one constant region of IgG each contain an Ig fold. An important step in the formation of an Ig fold is proline isomerization. While most peptide bonds have a trans conformation, proline's cyclic structure means that it is only slightly less stable in a cis conformation and can exist that that conformation in nature. A proline residue exists between two of the β-strands in the Ig fold, and this residue's isomerization from trans to cis conformation is often the rate-limiting step in the formation of an Ig fold.
Categories of Folding
[edit | edit source]While the final structures are generally very similar, Ig domains can broadly be grouped into three categories based on their folding processes.
In the first category, domains fold autonomously, guided by the internal disulfide bridge, and the final state is a monomer. One major intermediate exists between the unfolded polypeptide and its native folded state. This intermediate persists for a noticeable amount of time because it contains a cis proline residue, while in the final, folded state this residue is trans, and the transformation from cis to trans is relatively slow. The central β-strands in the intermediate are almost completely folded, and small helices link two pairs of the strands, making this intermediate highly stable. The reason for the high stability in the intermediate is because the small helices act as an organizing center in the antibodies and they position the bulky hydrophobic molecules in the center of the protein. These helices are found in the most commonly in the constant domains and not in the variable domains or in immunoglobulin molecules that are prone to misfold, suggesting that these internal linkages are key to the folding of the antibody. Examples of protein domains in this category include the constant region of the light chain (CL) and the second constant region of the heavy chain (CH2) of IgG.
The second category begins folding similarly to the first: it forms a partially-folded monomer, with a trans proline residue, which then isomerizes to a cis residue. At this point, the domain is unable to finish folding until it dimerizes with itself. A representative of this domain is the third constant region of the heavy chain (CH3) of IgG. In fully-formed IgG, the two heavy chains are directly joined at this domain (as opposed to CH2, which are separated by sugars), so its folding by this process makes sense.
The third category is more distinct from the first two. The first constant region of the heavy chain (CH1) of IgG is incapable of folding autonomously, and must associate with already-folded CL, forming a dimeric intermediate. As in the other two categories, the folding domain contains a trans proline residue that must become cis before folding is complete. Unlike the other two processes, this isomerization cannot occur until the CH bonds to the CL. This causes CH1 folding to be the slowest of all IgG domains; it does not happen until the heavy and light chains have already come together.
A commonality in the folding of every antibody is a slow proline isomerization reaction, which is the conversion from the trans proline to a cis proline. This reaction has a very high activation energy (~80 kJ/mol) which makes this a very slow reaction. Because of this fact, the proline isomerization reaction acts as a rate-limiting step in the folding of an antibody. In each of these three categories, there exist very important transition states. The rate-limiting proline isomerization reaction allows for the reactions to proceed slower and for the transition states to be populated since the proline must be in the cis conformation in order to proceed in the folding.
Quality Checks
[edit | edit source]B cells have been observed to go through a series of “quality checks” to in order to regulate the functionality of the antibodies being made. In the pre-B cell state, the folding of all the domains in the heavy chain is tested. After the heavy chain gene is rearranged, the pre-B cells produce IgMs, a short heavy chain with no light chains attached, bound to immunoglobulin heavy chain binding proteins (BiP). The BiP is bound to an unfolded CH1 domain, which is a part of the constant region in the heavy chain. A “surrogate” light chain is then produced from the variable domain of the pre-B cell. If the surrogate light chain induces the unbinding of the BiP with the IgM and the domain CH1 folds appropriately, then the heavy chain proceeds to the plasma membrane. If, however it fails this quality check, then the IgMs act as a substrate for endoplasmic reticulum associated degradation (ERAD). Thus the failure of this quality check leads to the degradation of the heavy chain. Then when the heavy chain is formed and a conventional light chain is formed, the same process as before takes place with the conventional light chains acting as the surrogate lights chains. The conventional light chains must induce the unbinding of the BiP from the CH1 domain and allow for folding in the CH1 domain of the heavy chains in order for the antibody to not undergo ERAD. Once the heavy chain and the light chain have gone through these two quality checks, disulfide bonds are formed between the heavy and light chains and the antibody is then ready for secretion.
Alternative States
[edit | edit source]Unlike most proteins, which can generally be said to exist in either a specific folded form or the denatured polypeptide form, antibodies are capable of forming some alternate structures under specific conditions. For example, below pH 3, antibodies can exist in a stable form unlike their typical one. While this is unlikely to directly cause any human health issues (a blood pH of 3 would be fatal long before misfolded antibodies caused any problems), it has ramifications for antibody production. Industrial antibody production includes steps that are carried out at low pH, which could affect the final product of the production.
Another, more direct problem that can come from alternative states of antibodies occurs when light chains or truncated heavy chains are secreted from B cells without forming a full antibody. These fragments can clump and become deposited in various organs, inhibited their function. The most common fatal complication from such deposition is light chain amyloidosis, in which monoclonal light chains are produced, secreted, and form deposits in the kidneys. The variable portion of the light chain is more likely to form amyloid deposits, possibly because the need for high variation in its structure make it more able to escape the cell without errors being detected.
Function
[edit | edit source]Antibody functions:
- Bind to foreign objects and prevent them from attacking normal cells
- Can help get rid of pathogens with the aid of macrophages
- Can directly damage pathogens by signaling the start of complement pathway, which is another immune response.
An example of antibody function would be in blood types. For instance, people with blood type A produce antibodies that recognize B antigens. If a person with blood type A was transfused with blood type B or blood type AB, the antibodies that recognize the B antigen on these blood cells would cause the person to begin clotting. This phenomenon explains why an individual of type AB blood can receive transfusion of type A or B blood however type A or B individuals cannot receive AB blood. This is the problem that usually occurs when blood donation is low and a match is necessary for medical purposes.
Five Major Classes of Antibodies:
1.) IgM :
a.This is a class of antibody that is produces after the initial exposure to antigen, but afterwards, its concentration in blood start to decrease. Immunoglobulin neutralizes the antigen and is responsible for the agglutination of the antigen. Because of this, it is very effective in complement activation.
b.IgM is a pentamer. It has µ heavy chains and exists as a pentamer in combination with another polypeptide called the J chain, which is responsible for initiating the polymerization to form the pentameric structure. With its large number of antigen-binding sites, each IgM molecule binds very tightly to any pathogen that has multiple copies of the same antigen on its surface. The binding induces the Fc region to activate the complement pathway which eventually causes the death of the pathogen. IgM also activates macrophages to phagocytose pathogens. Not surprisingly given these functions, IgM is the first antibody produced when an animal responds to a new antigen.
2.) IgG:
a.This class of antibody is present in tissue fluids. It is the most abundant class in bloodstream late in the primary immune response and particularly during the secondary immune response. IgG promotes neutralization, agglutination, and opsonization of the antigen, and it is also the only class of antibodies that can cross the placenta. It is secreted into the mother's milk and is taken up from the gut of the newborn animal into the bloodstream, thus it presents passive immunity to the fetus.
b.IgG is a monomer.
3.) IgA:
a.IgA presents the defense of mucous membranes by neutralizing the antigen, and also by agglutination. This antibody is present in bodily secretions such as saliva, tears, breast milk, mucus and in the secretions of the lungs and the intestine. IgA presents in breast milk also presents passive immunity on nursing infants.
b.IgA is a dimer.
4.) IgE:
a.Mast Cells, basophils of Histomine, and other chemicals that cause allergic reactions are released when IgE is triggered. IgE occurs in tissues where having bound the antigen. Some of these in turn activate white blood cells (called eosinophils) to kill various types of parasite. However, the mast cells can also release biologically active amines, including histamine, which cause dilation and increased permeability of blood vessels and lead to the symptoms seen in allergic reactions such as hay fever and asthma.
b.IgE is a monomer
5.) IgD:
a.In the antigen stimulated proliferation and differentiation of B Cells, IgD operates as the antigen receptor. This class of antigen exists on exterior of naïve B cells that have not been exposed to antigens.
b.IgD is a monomer.
Additionally, antibodies have proven to be remarkable because they can block specific protein synthesis within the body, while leaving human cells unharmed. This is due to the differences in ribosome structure found between bacteria and eukaryotic cells. The shapes of ribosomes in bacteria and humans have very specific differences that prove convenient when developing antibiotics meant to target just the bacterial ribosomes in order to halt their protein synthesis. If successful, antibiotics should bind and interfere with protein synthesis initiation complex formation in bacteria, or have some effect on the transcription of the bacteria's messenger RNA. Scientists trying to develop these antibiotics successfully take advantage of these structural differences in ribosomes, attempting to create ways to stop bacterial ribosomes from functioning without affecting the human in which they are located. For instance, mitochondrial cells contain ribosomes resembling those of bacteria, while the eurkaryotic cells hosting those mitochondria have much different ribosome structure. Thus antibiotics can target those bacterial-resembling ribosomes in the mitochondria without harming the eurkaryotic cell itself, which is merely one minor example that proves the remarkable role that structure and specificity can play in drug development.[1]
Antiviral Antibodies
[edit | edit source]Antibodies target the functional sites of the virus at hand. A failure of the antibody to target the functional site will usually result in the failure of the antibody to combat the variation of the virus. If the functional site cannot be targeted, the second choice target should be directed at the host molecules (not virus-encoded molecules). The more chemically similar the antibody and the target's natural ligands are, the more effective the antibody will be.
Generating Bispecific Antibodies
[edit | edit source]Antibody therapeutics are a valuable form of treating disease due in part to their selectivity for a target protein. However, certain drawbacks exist due to certain diseases having several different mechanisms. Bispecific antibodies (BsAb) create an interesting possible alternative with greater benefits. These antibodies can effective target two different binding sites. Some benefits include limited possibility of escape from therapy, greater tumor targeting, and more efficient cytotoxic selectivity. The main drawback for BsAbs is the difficulty in production. New technology has emerged in antibody production through single-chain variable fragment diabodies, tandem diabodies, two-in-one antibody, and dual variable domain antibodies. Although, these techniques have issues with too frequent dosing or conjugation problems.
CovX-Bodies present a technology based on the aldolase catalytic antibodies. This makes it possible to quickly generate the needed antibodies with proper targeting. A CovX body consists of two covalently bound pharmacophores. They're attached to the lysine at position 93 within the Fab arms of the scaffold antibody. They are created through a process of mixing a branched azetidinone linker with a peptide pharmacophore heterodimer with the aldolase antibody. The peptide is responsible for functional activities and the antibody acts to improve half-life and distribution properties. These antibodies are capable of being created with specific binding affinity, potency and pharmacokinetics. The CovX-Body, CVX-241 is currently taking advantage of angiopoietin-2 (Ang2), and vascular endothelial growth factor (VEGF). This drug has shown impressive phamacokinetics in rodents and nonhuman primates. Xenograft models have shown potent efficacy. CVX-241 is completing phase-1 clinical trials.
References
[edit | edit source]- ↑ National Institute of Health, "Inside the Cell", 2005, Pg.10
1. Buchner J, Feige M, Hendershot L. "How antibodies fold." Trends in Biochem. Sci. 35 (4): 189-198.
2. Colman, Peter M. "New Antivirals and Drug Resistance", The Walter and Eliza Hall Institute of Medical Research
3. David Hames, Nigel Hooper. Biochemistry.3rd edition. Taylor and Francis Group, New York, 2005.
4. Venkata R. Doppalapudi, et al. "Chemical Generation of Bispecific Antibodies." http://www.pnas.org/content/107/52/22611.full#ref-list-1. Richard A. Lerner. November 9, 2010. CovX. November 30, 2011.