Structural Biochemistry/MPS1 protein kinase inhibitor development
Discovery of the MPS1 gene
[edit | edit source]The MPS1 gene was found in a budding yeast, called the Saccharomyces cerevisiase. The gene (MPS1) after being cloned was at first named RPK1, but due to the MPS1 being the name that was used in the publication first, it stayed that way [1]. In the early years it was shown that MPS1 was a protein kinase by the use of glutathione S-transferase tagged MPS1, which resulted in strong autophsphorylation and substrate phosphorylation of other common kinase substrates. A phenotype analysis was done on the original yeast MPS1 mutants and as a result many critical functions were identified of the kinase.
MPS1 protein kinase function & importance in cancer
[edit | edit source]MPS1 protein kinases are involved in different steps in mitosis such as chromosome attachment and functions at the centrosomes. In addition, they participate in development and in multiple signaling pathways after mitosis. This large family of MPS1 protein kinases can be identified by a conserved C-terminal domain that appears in all the MPS1 protein kinases even if the N-terminal domain varies. The over-expression of MPS1 protein kinases in mitosis plays a role in cancer growth and has lead to the discovery and development of MPS1 protein kinase inhibitors.
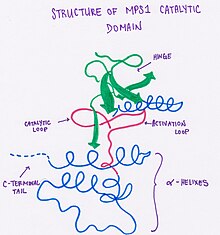
Structure of MPS1
[edit | edit source]This protein has a bilobe architecture, where the N-terminal is made up of a five-stranded β-sheet and an α-helix. In MPS1 specifically, there is an additional β-strand present. The two lobes of the protein are joined by Glu603-Gly605 (a hinge loop). The C-terminal is made up of a two-stranded β-sheet and next to this lobe are seven α-helixes, the catalytic loop and the activation loop. Polyethylene glycol (used in protein crystallization) was found to be present by the catalytic portion of the kinase and created a second pocket that isn't present in other kinases. This unique feature can be potentially used for the creation of inhibitors specific for this kinase.