Structural Biochemistry/Lysergic acid diethylamide
Background
[edit | edit source]Lysergic acid diethylamide, commonly known as LSD, has been known to cause psychedelic hallucinations and visions. It has not been known to be addictive or cause brain damage. LSD has been typically used for recreation in order to enhance creativity and out of body experiences. Albert Hoffman, in 1938, discovered LSD when making different derivatives of diethylamide. It's hallucinogenic properties were accidentally discovered in 1943. In the 1950s, LSD was brought to the medical community has a tool to create a temporary psychotic state and be used alongside psychotherapeutic treatments. It was in the 1960s that the general public began using LSD for recreational use, and remains a major drug to this day. [1].
Overview
[edit | edit source]LSD blocks serotonin from the brain. Serotonin, a neurotransmitter, is in charge of regulating mood, muscle contraction, and other cognitive functions. The reason why LSD blocks serotonin is because of their structural similarity, LSD is mistaken for serotonin and is then sent to the synaptic cleft instead of serotonin. And like many other hallucinogenics, LSD has a substituted indole ring in its molecular structure, which contributes to its hallucinogenic effects. There are many perceptual changes altering the cognitive and visual sensory systems, as well as changed in the sense of time, body-image, and ego. Memory is also greatly effected. A typical "trip" can last anywhere between six and ten hours. The half-life in humans is approximately 175 minutes, though it is metabolized at different rates at different structures in the body. Not much clinical testing has been done since the 1970's, partly due to the fact that after the subjects have taken a dose of LSD they become "too impaired to cooperate". [1]
Structure
[edit | edit source]The molecular configuration of crystal form LSD has been determined by using x-ray diffraction techniques. The configuration shows strain and steric hindrance. Serotonin and LSD are somewhat similar in structure as indicated by the indole ring present in both molecules. Therefore, the two molecules share similar chemical properties due to the similarity in structure. When comparing LSD and serotonin, the similarity between its electron densities of the highest occupied molecular orbital is observed. The nitrogen atom possesses the electron density due to its free lone pair.
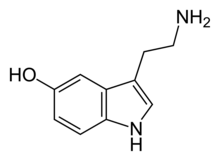
serotonin

LSD

LSD is prepared by reacting lysergic acid with diethylamide. LSD has the chemical composition C20H25N3O. It has four different isomers, however, only when it is R, and R, stereochemistry. Only one stereoisomer (the d-) is psychoactive. Therefore, a racemic mixture of LSD shows only half the potency of the dextro form. The electron density is lowest in the areas around the indole ring in both molecules. The dipole moment of the two molecules are very close. Serotonin is 2.98 D and LSD is 3.04 D; D is the SI unit measurement for electric dipole moment. The dipole moment is going towards the amine group in both molecules. The virtually same dipole moments of both molecules is the key to the ability of LSD fitting into the same receptors as serotonin.
Storage
[edit | edit source]LSD is a somewhat stable organic molecule, like other molecules with similar structures.The main factors to be concerned with are moisture due to chemical reactions in the presence of moisture, oxygen, light, and temperature. Reaction rates typically depend on temperature exponentially.
Synthesis
[edit | edit source]In its pure form, LSD is a white or clear, odorless, water-soluble crystal that can be crushed into a powder and dissolved. The most common form of LSD is known as blotter acid, sheets of paper that have are blotted with LSD. Tablet known as microdot is also very common. LSD is generally found as in its crystal form, dried on gelatin sheets or in capsule or liquid form.There a couple of different ways to make LSD. A common way is to start with lysergic acid. It will require morning glory seeds. Morning glory and the seeds contain lysergic acid amide. It is considered a precursor to LSD. The amount of LSA in different seeds varies . As a result, the quality of the drug made from it would also vary. Another synthetic route is to use ergot. Once the ergot is obtained, one must extract the ergot alkaloids which contain basic nitrogen atoms. A darkroom working environment is necessary because the ergot will decompose under bright lights. LSD itself can break down quickly when exposed to light.The solvents and reagents used in the synthesis are also harmful. The solvent anhydrous hydrazine can explode when heated. It is a carcinogen. Another chemical often used in for the synthesis is chloroform. It can cause cancer as well while causing internal damages to organs. The ergot alkaloid is synthesized into a compound called iso-lysergic acid hydrazide through the addition of reagents and heating. The iso-lysergic acid hydrazide is isomerized in order to make it active. Once cooled, the isomer is mixed with an acid and a base, and evaporated. The product obatined is iso-lysergic diethylamide, which is isomerized again to produce active LSD. The LSD is then purified and crystallized in order to obtain a higher concentrated form.
Side Effects of LSD
[edit | edit source]There have been no documented human deaths from an LSD overdose. It is physiologically well tolerated and there is no evidence for long-lasting physiological effects on the brain or other parts of the human organism. LSD may temporarily impair the ability to make sensible judgments and understand common dangers, thus making the user more susceptible to accidents and personal injury. It may cause temporary confusion, difficulty with abstract thinking, or signs of impaired memory and attention span