Structural Biochemistry/Lipids/Isoprenoids
General Information
[edit | edit source]
Also, referred to as terpenoids or prenol lipids, isoprenoids are any of a class of organic compounds composed of two or more units of hydrocarbons, with each unit consisting of five carbon atoms arranged in a specific pattern. These compounds are derived from five-carbon isoprene units and are biosynthesized from a common intermediate known as mevalonic acid, which is itself synthesized from acetyl-CoA. These lipids are considered to be the largest group of natural products, playing a wide variety of roles in physiological processes of plants and animals, and having a number of commercial uses.
In living organisms, these compounds range in function from pigmentation and fragrances to vitamins and precursors of sex hormones. Uses in industrial settings range from flavorings, solvents and raw materials for chemicals. These compounds are more commonly associated with being the main ingredients of perfumes and incense. But these are just a few of the many commercial uses for isoprenoids, which to this day are still expanding.
Isoprenoid Isolation and Identification
[edit | edit source]Isolation of isoprenoids from their natural sources is achieved through numerous procedures. Substances that are volatile and plentiful, such as turpentine, are best obtained by distillation of oleoresins. Rosin acids and fatty acids, which occur together in tall oil, are separated by fractional distillation at reduced pressure. Other compounds that are more on the rare side are best isolated by chromatography. A very laborious process known as enfleurage is employed for the isolation of heat-sensitive perfume ingredients. This process involves carefully placing the petals containing the oily isoprenoids in thin layers of purified fat to dissolve the oils. The oils are then recovered from the fat by washing the solution with alcohol.
Physical properties of an isoprenoid compound are the main factor that must be looked at to identify the best purification technique that will yield the best results. A heat-sensitive compound will not be efficiently and successfully purified with techniques such as distillation or sublimination, which require heat. Similar compounds in one source are best isolated and purified by treatment into a mixture of new substances that can be more readily separated. After separation, the original products can be attained by reconverting them. Generally, solid compounds can be purified by recrystallization and volatile compounds (solid or liquid) are best purified by distillation. Similar and nonvolatile substances might require chromatography to best achieve separation.

The determination of the elemental composition of isoprenoids is not a difficult task due to their simple hydrocarbon makeup and the availability of simple and reliable procedures for quantitative analysis of carbon and hydrogens. The presence of the other element in these compounds, oxygen, does not interfere with the analysis of the carbon and hydrogen makeup in a significant way, but does make it a more laborious task. A particularly difficult compound that was difficult to determine throughout the history of isoprenoid studies was camphor, which had a total of more than 30 different structures presented before the correct one was found.
Structures are more commonly identified by using nuclear magnetic resonance (NMR). This technique uses the help of a magnetic field to generate a response of the compound to this energy. This data is then collected and interpreted (computer analysis gives the best results to this day). Mass spectrometry, x-rays, and IR spectroscopy present other alternatives to NMR. High-resolution mass spectrometry enables the exact chemical formula of a compound to be determined, while X-ray crystallography permits the detailed spatial location of each atom to be determined from a diffraction pattern of the crystallized form of the structure.
Structural Features and Some Isoprenoid Compounds
[edit | edit source]The five-carbon unit that constitutes the basic building block of isoprenoids is a hydrocarbon called isoprene. This compound is a branched-chain unsaturated hydrocarbon, meaning it has one or more double bonds between carbon atoms. Isoprenoids can have one or more functional chemical groups attached to their carbon backbone, such as hydroxyls and carbonyls, which make up the diversity of isoprenoids. Isoprenoids can be classified as monoterpenes (C10H16) sesquiterpenes (C15H24), diterpenes (C20H32), triterpenes (C30H48), tetraterpenes (C40H64) or other polyterpenes (C5H8)n . Many isoprenoids are arranged in a “head to tail” manner in which a carbon atom of one isoprene unit is bonded to a carbon atom of the next isoprene unit. Triterpenes and tetraterpenes, though, will tend to bond its isoprene units in a “tail-to-tail” fashion.

Monoterpenes (C10H16)
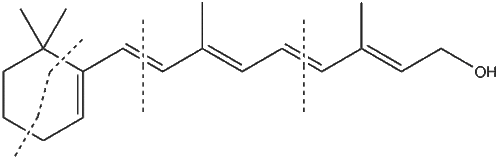
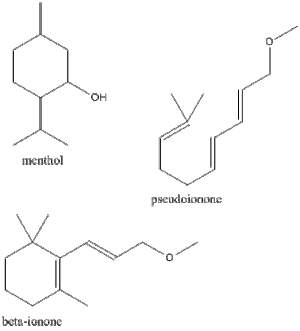
[edit | edit source]The first isoprenoids that were studied initially in the history of these lipids were the monoterpenes, the molecules of which contain 10 carbon atoms. These compounds are isolated from their natural sources by distillation of the plant matter with steam. They are characterized as volatile oils that are less dense than water and have normal boiling points in the range of 150 to 185ᵒC. Fractional distillation at reduced pressures work best at purifying these compounds. Some widely known monoterpene derivatives include the oxygenated acyclic monoterpene derivative citronellol and its corresponding aldehyde citronellal, which occur in oil of citronella. Others include the compound citral, which is found in lemongrass oil and geraniol, most commonly found in Turkish geranium oil.

The process used to convert citronellal is widely used commercially to supplement the natural sources of menthol, since this process produces a mixture of stereoisomeric menthols by catalytic hydrogenation. Citral is used in the production of rose-scented perfumes. The reduction of citral with sodium amalgam yields a compound called geraniol, which is responsible for the scent. Also, citral can be treated with acetone to condense it and yield pseudoionone, which is in turn treated with acid to produce β-ionone. Although not considered a terpene, β-ionone is commonly used as a starting material for the synthesis of vitamin A.
Sesquiterpenes (C15H24)
[edit | edit source]Sesquiterpenes are of lower volatility than monoterpenes and thus, can be isolated from their natural sources by extraction. Purification of these compounds involves either vacuum fractional distillation or chromatography. The oxygenated sesquiterpenes are the most commonly encountered ones. Sesquiterpenes may be acyclic or contain rings. A longer chain length and more double bonds contributes to a wide variety of cyclization structures. For example, there are two arrangements of isoprene units found in bicyclic sesquiterpenes: that of the cadalene types and the eudalene types. An example of each form is cadinene, the principal component of the optically active oils of cubeb and cade, which is of the cadalene type and β-Selinene, which is present in celery oil, and is of the eudalene type.


Diterpenes (C20H32)
[edit | edit source]Diterpenes are known to be antimicrobial and antiinflammatory. They derive from geranygeranyl pyrophosphate. One well known diterpene is phythol, an oxygenated acyclic diterpene that is the building block of the chlorophyll molecule. This compound is obtained on treatment with an alkali solution. Phythol is similar to vitamin A in terms of arrangement of their isoprene units (head-to-tail arrangement).

Another common diterpene is the commercially utilized tricyclic abietic acid. This compound is a carboxylic acid that constitutes the major portion of rosin. Rosin is the residue left after the isolation of turpentine and is the nonvolatile portion of the oleoresin of members of the pine family. It is used in the production of varnish and coating materials, among many other products. The versatility of this compound was discovered due to it being one of the cheapest organic acids available.

Triterpenes (C30H48)
[edit | edit source]An example of a triterpene is the acyclic hydrocarbon squalene (pictured above). This constitutes more than half of the liver oil of certain species of sharks and is otherwise, widely distributed in nature. Among shark liver oil, is it found in other fish liver oils, vegetable oils, fungi, and in human earwax. Squalene is a metabolic intermediate in the biosynthesis of cholesterol.
The most common types of triterpenes found in nature are those having five carbon rings. β-amyrin has usually been associated with much of the research done in the study of triterpenes. β-amyrin is found in the resin elemi, which is obtained primarily from trees in the family of flowering plants known as Burseraceae. Its skeletal structure shares many similarities with that of squalene and cholesterol.

Tetraterpenes (C40H64)
[edit | edit source]Some biologically important tetraterpenes include carotenoids, which account for the yellow, orange or red fat-soluble plant and animal pigments. Although they have eight lesser hydrogen atoms than the general formula of tetraterpenes, they are still considered tetraterpenes since they can be built up from isoprene units. Lycopene is the red pigment of the ripe tomato. It's structure has an interruption to the more common head-to-tail bonding of isoprene units. This interruption contains a single tail-to-tail attachment, which gives lycopene its symmetrical structure. Many tetraterpenes generally have this feature.

β-carotene is another more common tetraterpene, which is the yellow pigment of the carrot. This compound is a precursor to vitamin A, which means it is of nutritional importance since animals are able to cleave this compound at the point of symmetry to promote the production of active vitamin A. This vitamin is of considerable importance in the synthesis of pigments in the eye that are necessary for healthy vision.

Polyterpenes (C5H8)n
[edit | edit source]A well-known example of a polyterpene is rubber, in which n = 4,000-5,000. Oxidative degradation followed by x-ray diffraction revealed rubber to be made up of repeating units. The vulcanization of such a compound involves cross-linking between the chains through sulfur atom (disulfide bridges).

Biosynthesis
[edit | edit source]The synthesis of isoprenoids begins with acetyl coenzyme A, a compound derived from acetic acid and coenzyme A. In the first part of the process, mevalonic acid and isopentenyl pyrophosphate (IPPP) occur as important intermediates.

Afterwards, the formation of geranyl pyrophosphate (GPP, precursor of monoterpenes), occurs by the transformation of an IPPP to dimethylallyl pyrophosphate (DMAPP). DMAPP then combines with an IPPP to form this precursor.

Further combinations of the pyrophosphate compounds in a similar fashion can yield precursors to larger polyterpenes, such as the formation of squalene from GPP and IPPP, which can then form such isoprenoids as Lanosterol.
