Structural Biochemistry/Influenza Virus
Influenza Virus and Drug Design
[edit | edit source]The influenza virus is the main culprit of respiratory infection more commonly known as the "flu". The structure of the influenza virus includes a nucleoprotein (RNA) center enclosed in capsid, a lipid envelope, and spikes of two key proteins on its surface: hemagglutinin and neuraminidase. About 80% of the spikes consist of hemagglutinin. The function of hemagglutinin is to bind the virus with the host cell. It acts as to stick the virus onto the host cell in order to cause an infection. The other protein, called neuraminidase, cover the rest of the surface. The lesser amount of neuraminidase doesn't account for its large role, however. Neuraminidase helps facilitate the release of the newly formed viral molecules from the host cell. In perspective, hemagglutinin is the anchor that binds the virus to the host cell and neuraminidase is the trigger that induces a viral infection. Since neuraminidase is so important for influenza virus, scientists have developed methods to inhibit the protein and prevent influenza virus from infecting the host cell.
Structural based design has been one of the primary methods in the design of a drug. With the use of two key techniques, X-Ray Crystallography and Nuclear Magnetic Resonance (NMR), they have aided in the determination of a molecule’s structure. Such information as the three-dimensional structure of a specific target, helps direct drug creation because with structure based design, it allows one to observe the interaction between the target and the drug.
Recently, studies have shown a positive correlation between the similarity in drug and its target’s natural ligands to the increased barrier to resistance. In other words, the design of a drug needs to take into account that similarity, both physically and chemically, to the target’s natural ligands will make it more difficult for said disease and target to become resistant.
Neuraminidase Inhibitors
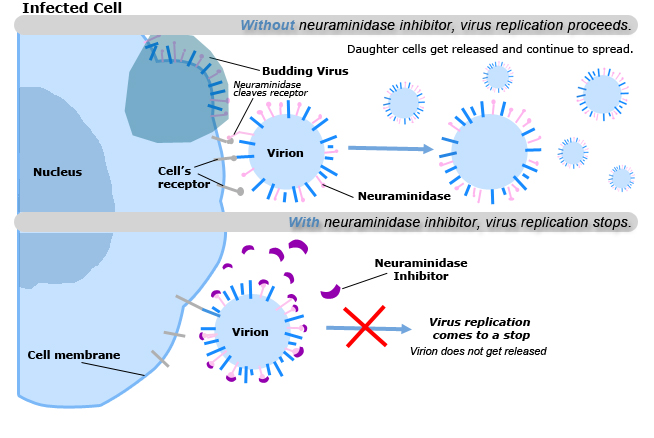
[edit | edit source]The discovery of neuraminidase inhibitors for the Influenza virus is one of the early examples of structure based drug design. The target of the Influenza drug is based on the virus’s neuraminidase enzyme. This enzyme’s role is to free the progeny of the virus in order to spread and infect other cells within the body. The neuraminidase inhibitors were created based on crystal structure data of the virus’s neuraminidase. What the neuraminidase inhibitor does is it chemically destroys the virus’s receptors thereby stopping virus replication. Neuraminidase inhibitors must be taken within 48 hours that symptoms arise. They do not ‘kill’ the flu virus, but merely slow down the virus replication to a point where the immune system can destroy it easier. As a result, they can reduce the severity and duration of a flu illness.
The spread of the flu virus is slowed in the body by the following steps: (1) The virus enters a cell with neuraminidase inhibitors present. (2) Once inside the cell, the neuraminidase inhibitors attach to the virus. (3) The flu virus can still use its host to replicate itself. (4) However, the inhibitors prevent the virus from leaving the cell—which halts the infection of other cells. (5)The virus is rendered ineffective and dies within the cell.
Current Antiviral Agents
[edit | edit source]Currently, ten neuraminidases enzymes have been discovered and their structures determined: N1-N9 for Influenza A and type B neuraminidase for type B Influenza. Four approved drugs that have been administered for public use are zanamivir, oseltamivir, amantadine, and rimantadine.
Zanamivir, oseltamivir, amantadine, and rimantadine are different in the types of influenza viruses they inhibit, route of administration, and approved use in age groups. However, the side effects and cost of zanamivir and oseltamivir vary from those of amantadine and rimantadine. Side effects, including nervousness, anxiety, concentration difficulty, and lightheadedness, have been reported to be less frequent in patients who take zanamivir and oseltamivir. In addition, zanamivir and oseltamivir are more expensive than rimatadine, which is more expensive than amantadine. While amantadine and rimantadine are drugs that have both been used extensively for treatment of influenza A, zanamivir and oseltamivir are new alternatives. Unlike zanamivir and oseltamivir, amantadine and rimantadine are chemically related antiviral drugs that act against influenza A viruses but not influenza B viruses. However, these antiviral agents for influenza are not a substitute for vaccine, but instead are a supplement.
Experiments have discovered that these two drugs have different resistances based on their method of intake and their binding affinity to the target. Oseltamivir is taken orally as a tablet whereas Zanamivir is taken as an inhalant. Oseltamivir is a prodrug, which means that it is inactive until it is taken within the body. Both drugs have been found that they work better according to those aforementioned methods. As for the inhibitors, amino or guandino groups have replaced the C4-hydroxyl groups of the neuraminidase enzymes. Changing the substituents optimize the binding potency both chemically and physically. According to Dr. Colman, “The loss of inhibitory potency toward neuraminidase…. zanamivir, and oseltamivir carboxylate, is greater the less the inhibitor resembles the substrate.” [1]
More studies have shown substantial evidence that support the hypothesis that drugs that resemble the target’s natural substrate and ligands are more successful at suppressing the possibility of drug-resistance. In fact, viruses need to be able to bind to its own substrates and to be able to distinguish against that of the drug. If drugs resemble the virus’s own substrates, it will be difficult for it to discriminate and therefore, become less resistant. In addition, this is why multi-drugs and multi-dosages are prescribed. Using “drug cocktails,” dosages, and routes of administration will result in better outcomes.
Inhibitors: Hemaggluttin and Sialidase
[edit | edit source]Two important proteins on the surface of influenza virus cells are lectin hemagglutinin protein and enzyme sialidase. Lectin hemagglutinin has 3 shallow sialic acid-binding sites while enzyme sialidase has an active site in a pocket. The deep active site of low molecular weight inhibitors makes sialidase the more attractive anti-influenza drug target than hemagglutinin.
Influenza virus binds to sialic residues. Because of this, viruses gain entry into the host cell by adhering to the surface of carbohydrates. The viral protein that binds to sugars is called hemagglutinin. Once the virus penetrates the cell membrane, a viral protein called neuraminidase cleaves the glycosidic bonds of the sialic acid residues, freeing the virus to infect the host cell. Neuraminidase inhibitors are analogues of sialic acid that block the active site of neuraminidase and leave uncleaved sialic acid residues on the surface of host cells and influenza viral envelopes. Viral hemaggluttin binds to the uncleaved sialic residues, reducing the spread of infection to other cells.
Notes
[edit | edit source]- ↑ Colman, Peter M. "New Antivirals and Drug Resistance", 'Annual Review of Biochemistry', 2009.
References
[edit | edit source]- Colman, Peter M. "New Antivirals and Drug Resistance", 'Annual Review of Biochemistry', 2009.