Structural Biochemistry/Glutamine Addiction in Cancer
Introduction
[edit | edit source]Many cancers survive and grow based on the rate of aerobic glycolysis. Some cancers are attracted to glutamine, one of the 20 common amino acids that code for a genetic sequence or code. However, glutamine is not a typical amino acid that synthesizes glucose used in the process of glycolysis. Moreover, cells uptake glutamine not because it is a nitrogen donor to nucleotides. In fact, glutamine plays an important role in up-taking essential amino acids and activating TOR kinase, which is a specific enzyme that is necessary for balancing protein synthesis and degradation. Glutamine is the main mitochondrial substrate that is required to maintain the membrane potential in mitochondria of cancer cells as well as aid in NADPH production that is necessary for synthesizing other macromolecules as well as control redox chemical reactions within the body.
Addiction to Glutamine
[edit | edit source]
It has been studied by Otto Warburg, a notable German physiologist and Nobel Laureate, that cancer cells seem to uptake more glucose and produce more lactic acid than regular cells or tissues. Warburg hypothesized that cancer results from falling back to metabolism that involves rapidly increasing the number of single cell eukaryotes. This became known as the Warburg effect and studies have shown that this effect is brought on by activating oncogenes associated with glucose uptake. Furthermore, activation of the cell signal, phosphoinositide 3-kinase (PI3K) cause levels of glucose uptake to increase and causes the cell's metabolism to exceed the maximum use for glucose. Because of this, cancer cells can then secrete extra glycolytic metabolites in the form of lactic acid. Some tumors have this similar reaction, but instead of excess glucose metabolism, there is inefficient glutamine metabolism. These types of cancer cells or tumors cannot survive when there is not enough extra glutamine and are therefore considered to be "addicted" to glutamine. Glutamine is in fact a necessary substrate used in anabolic growth of cells, especially those of mammals. Due to the addiction of glutamine exhibited by some cancer cells, the study of glutamine in cell growth and cell-signaling pathways will help to discover new therapeutic treatments of some cancers.
Glutamine as a Nitrogen Donor
[edit | edit source]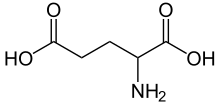
Cancer cells, like any other cell, must synthesize compounds that contain nitrogen. Typically these compounds are in the form of nucleotides and essential amino acids. Glutamine is a typical nitrogen donor and donates through three enzymatic steps in the synthesis of purines and two enzymatic steps in the synthesis of pyrimidines. Glutamine donates an amide group and is then converted to glutamic acid.
Glutamic acid becomes the primary donor of nitrogen for synthesizing nonessential amino acids. Transaminases are specific enzyme that aid in transferring the amino group of glutamic acid to α-ketoacids which are used to create nonessential amino acids. Some examples of α-ketoacids include pyruvate, oxaloacetate, or gamma-semialdehyde which are also used to synthesize nonessential acids such as alanine, serine or aspartate. Glutamic acid as a form of glutamine, donates its carbon skeleton and nitrogen to proline, another nonessential amino acid, as well.
Activation of TORC1 Protein
[edit | edit source]
Glutamine plays an important role in the process of protein translation in cancer cells. This was observed in the response to glutamine of target rapamycin complex 1 in mammals (mTORC1). mTORC1 is typically a major regulator of cell growth and activates protein translation but inhibits the response of macrophages to excess amino acid production. Through the study of yeast, it was concluded that there needs to be a sufficient level of amino acids for TORC1 to be properly activated. TORC1 seemed to respond to glutamine as well as essential amino acid levels. mTORC1 activation seems to response mostly to leucine; however glutamine has been shown to be necessary to activate mTORC1 to the maximum. Studies of cell lines of mTORC1 showed that cells depended not only on the presence of essential amino acids but also glutamine at the same time. From studying how glutamine is taken up through an importer called SLC1A5 showed that glutamine was exported using SLC7A5 which exchanges glutamine for uptake of essential amino acids. If there was not enough SLC1A5 in the cells, glutamine could not be taken up and exported; therefore essential amino acids could not be absorbed either. Glutamine also contributes to nonessential amino acids that are found in proteins that are newly translated.Therefore, it could be concluded that glutamine is not used for anabolic metabolism but instead for exchanging essential amino acids that activate TORC1 used in translating proteins, in and out of the cell. Ultimately, glutamine signals mTORC1 as well as provides essential amino acids for protein translation.
Mitochondrial Substrate: Glutamine
[edit | edit source]In 1955 Harry Eagle found that glutamine was essential for proliferating cells. Eagle studied on nutritional needs of a cell and found that glutamine was consumed ten times more than any other amino acids. Cells were unable to multiply without glutamine. Kovacevic and collegues found, in 1971, that glutamine is used as fuel and the carbon molecules found in gluatmine was also found in the carbon dioxide excreted by the cell. Glutamine loses its amide group using the an enzyme, glutaminase, to produce glutamic acid, which then loses its amine group using glutamic acid dehydrogenase to form α-ketogluterate.
Current studies use 13C to identify the carbon movements in the conversion of glutamine to lactic acid. Gluatamine uses malic enzymes to convert to lactic acid. Malic enzymes decarboxylates malic acid making carbon dioxide, NADPH and pyruvate. The NADPH produced is then used to for the cell to multiply.
Glutamine also contributes to the cell by producing oxaloacetate according to studies shown in a glioblastoma cell. The oxaloacetate (OAA) is bonded to an acetyl-CoA to make citrate. The acetyl-CoA is formed by breaking down cholesterol, fatty acids and chromatins. Gluatmine goes through anaplerotic reactions to refill the amount of carbons entering the TCA cycle. By replenishing the carbons in the mitochondria the cell is able to synthesize nucleotides, proteins, and lipids. Due to glutamine metabolism in cancer cells, studies show that the mitochondria in essential even for cancer cells. 13C NMR studies do show that cancer cells do not depend of oxaloacetate production through pyruvate carboxylation. In fact cancer cells suppress the activity of pyruvate carboxylation since cancer cells have glutamine to produce oxaloacetate.
Glutamine Metabolism Regulated by Oncogenic Levels of c-MYC
[edit | edit source]As discussed above, glutamine serves as a crucial nitrogen donor for the purpose of nucleotide synthesis. Studies that utilize reverse transcriptase ((RT)-PCR), and chromatin immunoprecipitation, suggest that the c-MYC (Myc) is involved in activating 11 genes that are involved in nucleotide biosynthesis. Massive amounts of Myc have been linked to the increase process of glutaminolysis, which suggests that Myc activation and amplification serves as one of the more common oncogenic events that can be witnessed in some cancers. Rt-PCR and ChIP both have the tendency to advocate the binding of Myc and transcriptional occurrences of two glutamine transporters: the SLC38A5 (SN2) and SLC1A5 (ASCT2). SLC1A5 is the transporter that is necessary for the glutamine-dependent activation of mTORC1. Myc also serves as a promotion factor to the metabolism process of converting glutamine into glutamic acid, which eventually becomes lactic acid at the end of the metabolism process. In addition, Myc also plays a significant role in influencing the post-transcriptional regulation of glutamine catabolism. The Myc is also a factor that leads the mitochondrial membrane to be dependent on exogenous glutamine as a source of carbon. Despite the fact that there is a great availability of glucose, glutamine depletion within Myc-transformed cells also significantly decrease the levels of TCA cycle metabolites.
Ways to Target Cancer
[edit | edit source]Many cells are sensitive when they are deprived of glutamine especially cancer cells in pancreatic cancer, glioblastoma multiforme, acute myelogenous leukemia and lung cancer. In the 1950s, experiments were performed and showed that 6-diazo-5-oxo-L-norleucine, azaserine and acivicin were analogs of glutamine. Testing of these analogs showed that they have an effect against certain tumors. These compounds inhibited enzymatic steps that involved glutamine when synthesizing nucleotides. The following are current ways to reduce cancer that have been researched and studied:
1. Glutamine uptake suppression
Studies show that there has been an increase transporters that have great attraction or affinity to glutamine in cancer cells specifically. A common transporter of glutamine is known as SLC1A5 and targets the Myc oncoprotein. Many cancers display an increase in this transporter; however there are inhibitors that have been discovered that allow the glutamine to be taken up less or at least not in excess amount. One of these inhibitors is called GPNA or L-γ-glutamyl-p-nitroanilide and it not only inhibits the amount of glutamine that is absorbed, but it also causes the mTORC activation, but only those proteins that depend solely on glutamine, to be suppressed as well.
2. Transamination Suppression

Because the major route that allows carbons derived from glutamine to enter the citric acid cycle is through a process known as transamination, studies have shown that the transaminase inhibitor known as amino-oxyacetic acid (AOA) can help with suppressing cancer. Treatment of AOA seemed to be promising since it produced a cytostatic effect where it inhibited cell growth in breast cancer. It also showed the same effect on cell growth in glioblastoma cells. Inhibiting one component of glutamine metabolism or in this case glutamate transamination resulted in the reduction of cancer effects.
3. Inhibition of Complex I in Citric Acid Cycle

Cancer cells that depend on glutamine cause the mitochondria to produce anabolic precursors using glutamine instead of glucose. Glutamine will flow in and out of the citric acid cycle and will cause NAD+ to be regenerated continuously using the electron transport chain. Drugs have been developed to suppress these effects in the mitochondria of cells. For example, Metformin has been proven to slow the growth of cancer cells and tumors and displays efficiency in the mitochondria of liver cells. Metformin not only targets glutamine metabolism but also lowers blood glucose concentration.
4. Stopping mTORC Activation
Glutamine can be imported into cancer cells through the transporter SLC1A5. This also allowed essential amino acids to be imported such as leucine, which will activate mTORC1 kinase. Treating cancer cells with GPNA which inhibits SLC1A5 blocks glutamine from activating mTORC1 and causes autophagocytosis of cancer cells to occur.
5. Lower Blood Glutamine Levels
Enzymes can be used to lower the blood glutamine levels. Asparagine which hydrolyzes into aspartic acid through the help of the enzyme L-asparaginase can be used to treat acute lymphoblastic leukemia. All of the cells can synthesize asparagine and thus L-asparaginase can cause glutamine to hydrolyze into glutamic acid and ammonia as well. L-asparaginase lowers glutamine levels; however it can also have a high toxicity level.
Alternatively, research has been further conducted to discover that plasma glutamine can be decreased by using phenyl butyrate. This has been specifically used to treat hyperammonemia patients that have acute liver failure or urea cycle disorders. Phenyl butyrate has been successful in decreasing the amount of glutamine in cells and can break down to form phenyl acetate that can combine with the leftover glutamine with the aid of phenyl acetylcoA to form phenylacetylglutamine which can be simply excreted from the body through urination; thus reducing the amount of glutamine in the body and reducing the attraction of cancer cells to glutamine.
Conclusion
[edit | edit source]Certain oncoproteins in tumor cells can alter the metabolism of tumor cells making them have a higher affinity to the amino acid glutamine. Initially this came as a bit of a surprise considering that glutamine is a non-essential amino acid that can easily be synthesized in the cell. However, after considering the role that glutamine plays in metabolism and in affecting cell growth, it became clear that glutamine was ideally suited to further the goal of cancerous cells. By taking advantage of the fact that some cancerous cells may be addicted to glutamine, researchers can perhaps develop therapeutic treatments to eliminate this special class of tumor cells. The challenge, however, is to create a drug that can target the glutamine used in the cancer cells, but leave the glutamine in normal, non-transformed cells untouched.
Reference
[edit | edit source]Wise, David R. and Thompson, Craig B. "Glutamine Addiction: A new Therapeutic Target in Cancer" Trends Biochem Sci. 2010 Aug; 35(8):427-33. Review.