Organic Chemistry/Cycloalkanes
Overview
[edit | edit source]A cycloalkane is a regular alkane with a ring or loop. An example is cyclohexane, which is a ring of 6 carbon atoms, each bonded to 2 hydrogen atoms (C6H12).
We briefly discussed cycloalkanes in the alkanes unit of this book, but in this unit, we'll be going into much greater detail about cycloalkanes as well as cycloalkenes. There are a number of properties that are unique to these structures and thus they deserve special attention.
Because of their cyclical nature, cycloalkanes do not have the freedom of rotation that regular alkanes possess. Stereochemistry plays a very important role in both the limitations of movements, but also, in some instances the limitations of reactions that can take place. Often these limitations are due less to the cyclical nature itself so much as the lack of freedom of rotation found in such alkanes.
A good example is the restrictions placed on E2 elimination reactions, which we'll cover later in the book.
Drawing Cycloalkanes
[edit | edit source]
The above image of the first four simple cycloalkanes shows that each carbon has 2 hydrogens attached. As you can see, the other 2 bonds are to adjacent carbons. While the shapes show the basic 2-dimensionaly, top-down shape, only cyclopropane and cyclobutane have all of their carbons on a plane. Cylcopentane and cyclohexane, as well as higher cycloalkanes, all have a 3-dimensional geometry. Cyclohexane is particularly important and it will be discussed separately.

More often, cycloalkanes, like many other organic structures, are drawn such that their carbons and hydrogens aren't labeled. Cyclopentane and cyclohexane (see above) are generally drawn as a pentagon and a hexagon, respectively.
Naming of Cycloalkanes
[edit | edit source]Naming of cycloalkanes is similar to alkanes. The smallest cycloalkane is "Cyclopropane", so as you might imagine, this is a ring of 3 carbons. If the number of carbons on the cycloalkane is higher than the number of carbons on any of its substituents (that is, carbon chains attached to carbons on the cycloalkane), then the base name is prefixed with "cyclo" and the rest of the name the same as an alkane with the same number of carbons.

If, as in the above image, the cycloalkane has fewer carbons than a carbon chain it's connected to, then the longest carbon chain is the primary and the cyclic portion receives the substituent "-yl" ending and prefixes the primary name. Thus the name 1-ethylbutylcyclopentane, while technically an accurate description, is not the correct name.

Numbering of carbons in substituted cycloalkanes is fairly straight-forward. If there is only one substituent, it is the 1-carbon. If there are more than one, then numbering proceeds, clockwise or counter-clockwise, such that the lowest number after 1 goes to the least (or a least) substituted carbon. I know that sounds tricky, so we'll look at the example on the left, 3-chloro-1,1-dimethylcyclohexane. the carbon with the chlorine bond is the least substituted carbon, so it gets the lowest number after 1 and 1 goes to the nearest carbon with more than 1 substituent. So, the 1 is applied to the methyls. Substituents are still placed in alphabetic order, as you can see from the second example, 2-bromo-3-chloro-1,1-dimethylcyclohexane.
Ring Strain in Cycloalkanes
[edit | edit source]Carbons with 4 single bonds want the bonds to be at a tetrahedral angle of 109.5°. In 1885, Adolf von Baeyer proposed that since carbons prefer this angle, that only rings of 5 and 6 members should be possible. Baeyer made the mistake of thinking in 2-dimensions and assuming that all rings are planar. In fact, only cyclopropane and cyclobutane are flat, resulting in their bond angles of 60° and 90°, respectively. But for some time, it was believed that these smaller cycloalkanes could not be created and that ones of more than 6 carbons would have strain because their angles would be too far in excess of the 109.5° preferred angle.

In the above figure, the first four simple cycloalkanes are again represented, but this time the 3 dimensional representations are shown. Notice how cyclopentane has 4 carbons on a plane and the 5th is slightly off the plane, giving it the shape of an open envelope.
In fact, rings larger than 3 carbons have 3 dimensional shapes that relieve this bond strain, but it's important to note that bond strain does affect stability. For cyclopropane and cyclobutane, the strain energy is about 110 kJ/mol. Cyclobutane can enter a "puckered" formation that slightly relieves some torsional strain. Cyclopentane, which is non-planar can remove some of the strain and has only about 25 kJ/mol of strain. Cyclohexane, because of its chair conformation, can maintain perfect tetrahedral angles, resulting in no strain. As the ring size goes up further, angle strain can be avoided at the cost of introducing some eclipsing strain, and vice versa, so that some strain exists, but the most strained of these, cyclononane, has only about 50 kJ/mol of strain. Ring strain pervades for ring sizes up to 13 members. After that, there are enough carbons that ring strain is removed completely.

But angle strain isn't the only issue involved in ring strain. Torsional strain is also a factor. When the hydrogen bonds in a ring eclipse each other, this creates additional strain. In the case of cyclopropane the hydrogens eclipse each other, greatly adding to the strain. In fact, this is the cause of most of the strain found in cyclopentane. Cyclopentane has bond angles very close to 109.5°, but because 4 of its carbons are planar, there it has torsional strain that makes it less stable. Looking at the cyclopentane along 2 of the bonds on the plane, with the non-planar carbon closest to us, in the figure above, one can see that the bend in the bonds allows the hydrogens to move slightly out eclipse, reducing the torsional strain somewhat.
Cyclohexane is the lowest membered ring that's able to have both 109.5° bond angles and maintain a staggered conformation, allowing it to be strain free.
Conformations of Cyclohexane
[edit | edit source]There are 3 main conformations of Cyclohexane, the chair, the reverse chair and the boat. The chair conformations have no angular, torsional or steric strain. However the boat conformation does have steric strain, as the hydrogens at the high points on the front and back of the boat are slightly overlapping.
Conformations of Mono- and Di-substituted Cyclohexane
[edit | edit source]TO DO
The Effect of Conformation on Reactions with Cyclohexane
[edit | edit source]Different conformations of cyclohexane can react differently for certain reactions. For example, the E2 elimination reaction, which we'll cover later in this book, requires that a halogen atom and a hydrogen atom be on adjacent carbons and coplanar from each other, or 180° (anti). In cyclohexanes with multiple substituents attached to its carbons, it's possible for that the hydrogen and halogen can be on adjacent carbons, but never be coplanar, thus preventing the reaction from occurring.
In other reactions, steric interference from adjacent substituents, for example, a large alkyl group on an adjacent carbon, can prevent a nucleophile from attacking a desired location. For these, and other reasons, it's important to understand the 3-dimensional geometry and structure of the reactants as well as the mechanisms of the reactions that are taking place.
Large Ring Systems
[edit | edit source]TO DO
Polycyclic Ring Systems
[edit | edit source]In some compounds, two or more rings are fused together in the same molecule. This can occur in both aliphatic and aromatic compounds.
If the two rings meet at a single carbon atom, where that carbon atom is part of both rings, the compound is called a spiro compound. The two rings are roughly (but not exactly) perpendicular to each other because of the usual tetrahedral bonding of four ligands around carbon.
If the two rings are bridged at two or more carbon atoms, the carbon atoms form part of a bridge, and the compound is a bicycloalkane (for two rings) or a polycycloalkane (for three or more rings).
An aromatic bicyclic compound is naphthalene (C10H8), which is a pair of benzene rings fused across a carbon-carbon bond. An aliphatic (non-aromatic) bicyclic compound is decalin (C10H18), formally known as decahydronaphthalene, which is a pair of cyclohexane rings fused across a C-C bond.
Naming conventions for bicyclic rings
[edit | edit source]A bicyclic alkane ring system is composed of three parts:
- The longer carbon chain (a)
- The shorter carbon chain (b)
- The carbon-carbon bridge (c)
Note that always.
A bicyclic ring is named according to the total number of carbons in the following form:
- bicyclo[a.b.c]alkane
In this formula, substitute [a.b.c] with the lengths of the carbon chains, and substitute "alkane" with the total number of carbons (heptane, octane, nonane, etc.).
A famous example of a bicyclic compound is norbornane (see image below). The formal name for norbornane is bicyclo[2.2.1]heptane. There are two carbons to the left of the bridge, two carbons to the right of the bridge, and one carbon on the bridge itself. Therefore, the numbers in the brackets are [2.2.1]. Note that the two carbons at the base of the bridge are not counted in the brackets.
Introduction to Heterocyclic Compounds
[edit | edit source]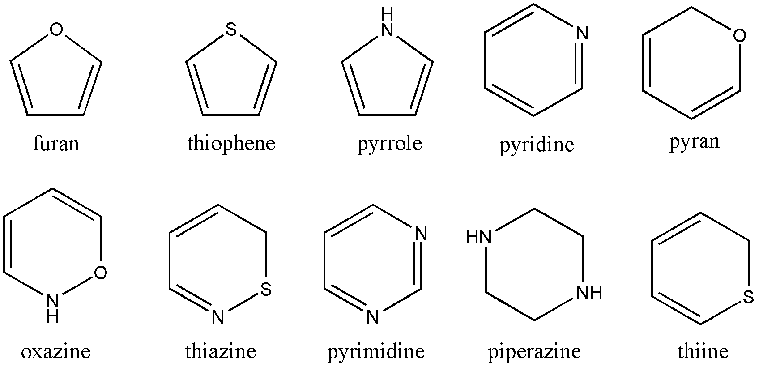
Heterocyclic compounds are cyclic compounds that contain atoms other than carbon in their ring. The figure above shows just a few single-ring heterocyclic compounds. The ones shown are simply 5 and 6 membered rings. There are smaller and larger rings and there are also multiple ring heterocycles.
Heterocyclic compounds play a big role in organic chemistry and because they have different electron configurations from carbon, they react differently from carbon rings and differently from each other. Later in this book we will discuss several specific heterocyclic compounds that are very commonly used in organic chemistry and we'll discuss their individual characteristics.
For now, it's simply important to know what a heterocyclic compound is.
![]() || << Haloalkanes | Alkanes | Alcohols >>
|| << Haloalkanes | Alkanes | Alcohols >>

