Organic Chemistry/Chirality/Diastereomers
Diastereomers
[edit | edit source]Diastereomers are stereoisomers that are not enantiomers (mirror images) of each other. Due to their different shape, diastereomers can have different physical and chemical properties. This is perhaps especially true of diastereomers involved in biological systems.
According to IUPAC the term "geometric isomerism" is an obsolete synonym of "cis-trans isomerism" and its use is strongly discouraged. Sometimes the term "geometric isomerism" has been used as a synonym of stereoisomerism, i.e. optical isomers being considered to be geometric isomers. This, however, is not consistent with current standard chemical nomenclature. The exact term for stereoisomers that are not optical isomers is diastereomers.
A special kind of diastereomer is an epimer. Epimers are diastereomers that differ at one of several asymmetric carbon atoms. There is also something called an anomer, a special type of epimer. An anomer differs at a new asymmetric carbon atom when a ring is formed (in carbohydrate chemistry).
Cis-trans Isomerism
[edit | edit source]Stereoisomerism can occur when a double bond is present, because the pi bond involved prevents that bond from being "twisted" the same way that a single bond can be. A good example is 1,2-dichloroethene: C2H2Cl2. Consider the two examples below:
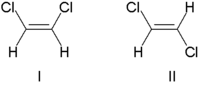
The two molecules shown above are cis-1,2-dichloroethene and trans-1,2-dichloroethene. These two molecules are geometrical isomers because the two carbon atoms cannot be rotated relative to each other, due to the rigidity caused by the pi bond between them. Therefore, they are not "superimposeable" – they are not identical, and cannot take each other's place. Cis/trans isomers have different chemical and physical properties and can exhibit dramatically different biological activity.
Cis-trans isomerism (Often called geometric isomerism although this term refers to all stereoisomers) is a form of stereoisomerism and describes the orientation of functional groups at the ends of a bond around which no rotation is possible. Both alkenes and cycloalkanes have restricted rotation around certain bonds. In alkenes, the double bond restricts movement and rotation, as does the looped structure of cycloalkanes.
Rotation is possible around the double bond of an alkene but it requires between 60 and 70 kcal of energy. Without the addition of this energy, groups that start on one side of the double bond stay there. This is the basis of cis/trans isomerism.
There are two forms; the cis and trans isomers. The form in which the substituent hydrogen atoms are on the same side of the bond that doesn't allow rotation is called cis; the form in which the substituent hydrogens are on opposite sides of the bond is called trans. An example of a small hydrocarbon displaying cis-trans isomerism is 2-butene.
Cis isomers and trans isomers of a substance have different physical properties. Trans isomers generally have higher boiling points and lower densities. This is because the trans isomers molecules can line up and fit together better than the cis form. Two isomers with very different properties are maleic acid and fumaric acid. The names are two trivial names for 2-butenedioic acid and respectively the cis and trans isomer.
Cycloalkanes and similar compounds can also display cis-trans isomerism. As an example of a geometric isomer due to a ring structure, consider 1,2-dichlorocyclohexane. These compounds can be named more rigorously using R/S notation.

|

|
| cis-1,2-dichlorocyclohexane | trans-1,2-dichlorocyclohexane |
| 1(R),2(S)-dichlorocyclohexane | 1(S),2(S)-dichlorocyclohexane |
E/Z notation
[edit | edit source]Main article: E-Z System
The trans/cis system for naming isomers breaks down when there are more than two different substituents on a double bond. (The cis/trans system should only be used when the carbon atoms involved each have a hydrogen atoms attached). The E/Z notation is unambiguous. Z (from the German zusammen) means together and usually corresponds to the term cis; E (from the German entgegen) means opposite and usually corresponds to the term trans.
Usually, E isomers are more stable than Z isomers because of steric effects. When two large groups are closer to each other, as they often are with Z, they interfere more with each other and have a higher potential energy than with E, where the large groups are farther apart and interfere less with each other.
Diastereomers with stereocenters
[edit | edit source]In simple terms, two stereoisomers are diastereoisomers of each other if only one chiral center differs between the two stereoisomers. That is to say, if both molecules contain two or more chiral centers, but if only one of the chiral centers in each molecule is different than the other, then the two molecules are diastereoisomers of one another.
If a molecule contains a single asymmetric carbon atom or stereocenter, it will have two mirror image forms. If a molecule contains two asymmetric carbons, there are four possible configurations, and it would be mathematically and physically impossible for all four to be mirror images of each other. The more chiral centers in a molecule, the more possibilities there are for different conformers, and therefore the more possible diastereomers exist.
As an example, tartaric acid contains two asymmetric centers, but two of the configurations of the tartaric acid molecule are equivalent to one another -- and together they are called meso compounds. This configuration is not optically active, while the remaining two configurations are D- and L- mirror images. For this reason, the meso form of tartaric acid is a diastereomer of the other forms.
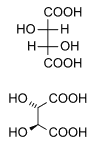
|

| |
|
(natural) tartaric acid |
D-(-)-tartaric acid |
mesotartaric acid |
|
(1:1) |
||
Two common prefixes used to distinguish diastereomers are threo and erythro. When drawn in the Fischer projection the erythro isomer has two identical substituents on the same side and the threo isomer has them on opposite sites.
Carbohydrates
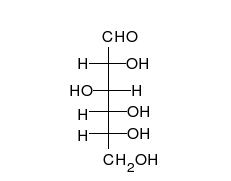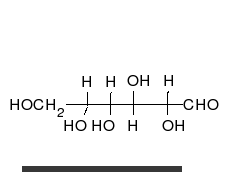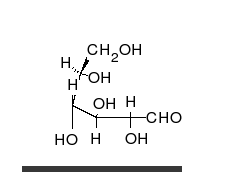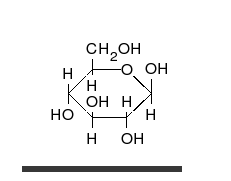
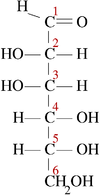
[edit | edit source]The families of 5 and 6 carbon carbohydrates contain many diastereomers because of the large numbers of asymmetric centers in these molecules. Since each carbon in the primary chain of an aldose (one type of carbohydrate) and all but one of the carbons in the primary chain of a ketose (another type of carbohydrate) have both a hydrogen and a hydroxyl group attached, most of the carbons in any given sugar are actually chiral. Since the number of possible conformers for a chiral molecule is 2 raised to the n power (2n), where n is the number of chiral centers, this makes for a great deal of variability in carbohydrates and a large number of diastereomers.

|

|

|
|
| D-glucose | L-glucose | D-galactose | D-mannose |
Glucose adopts a ring structure in solution. This is super awkward to show with a Fischer projection so a Haworth projection is usually used instead:
-OH groups on the right of a Fischer projection are drawn below the ring in the Haworth projection.
Diastereoselectivity
[edit | edit source]Diastereoselectivity is the preference for the formation of one or more than one diastereomer over the other in an organic reaction.