Climatology/Atmospheric Composition and Structure

Meaning of Composition of Atmosphere
[edit | edit source]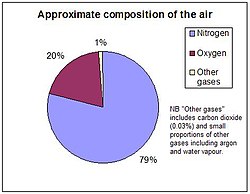
The literal meaning of composition is ‘ingredients’ or ‘constituents’ of something. In another words, it is a manner by which something is made up of. When we apply the same meaning with atmosphere, it signify the items or the elements with which our atmosphere is composed. Our atmosphere is composed of numerous gasesand other substances, hence, it is a mechanical mixture of the gases, water vapour and dust particles. Let us discuss about the composition of atmosphere.
Composition of Atmosphere
[edit | edit source]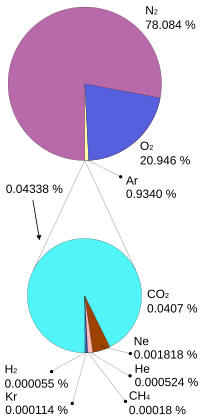
The envelope of atmosphere around the earth,a mechanical mixtures of numerous gases and other substancesare very important to all living organisms of the planet. The four major gases – nitrogen, oxygen, argon and carbon dioxide together constitute 99.99% of the total volume of dry air. The maximum concentration is of nitrogen with more than 78 percent while the oxygen is a little less than 21 percent.
| Gas | Volume(A) | ||
|---|---|---|---|
| Name | Formula | in ppmv(B) | in % |
| Nitrogen | N2 | 780,840 | 78.084 |
| Oxygen | O2 | 209,460 | 20.946 |
| Argon | Ar | 9,340 | 0.9340 |
| Carbon dioxide | Template:CO2 | 413.32 | 0.041332 |
| Neon | Ne | 18.18 | 0.001818 |
| Helium | He | 5.24 | 0.000524 |
| Methane | CH4 | 1.87 | 0.000187 |
| Krypton | Kr | 1.14 | 0.000114 |
| Not included in above dry atmosphere: | |||
| Water vapor(C) | H2O | 0–30,000(D) | 0–3%(D) |
| notes: (A) volume fraction is equal to mole fraction for ideal gas only, | |||
Nitrogen
[edit | edit source]Nitrogen is the most abundant found in atmosphere constituting 78.084 percent to the total volume of dry gases. This is almost chemically inactive and have nothing to do with any sort of chemical actions in the atmosphere. It does not combine freely with other elements, hence, it is termed as neutral substance. This gas is found beyond a height of 100 km, but its concentration is below 50 km height from the sea level. This gas is significant for the growth and reproduction in plants and animals. Certain bacteria in the soil are capable of converting a very small amount of atmospheric nitrogen into nitrates and fix it to the soils and water bodies to be consumed by animals and plants. This process is called as Nitrogen fixation. The nitrogen fixed in the earth’s surface is again converted and sent back to the atmosphere by bacterial action through a chemical reaction called denitrification.
Oxygen
[edit | edit source]It is the second largest gas of the atmosphere constituting 20.9476 percent of the total dry atmospheric gases. It is very essential for the survival of many of the living organisms of this planet. It is chemically very active gas. It is combined with several other elements and forms varied compounds. Oxygen is vital for combustion of fuels. When anything burns, oxygen is consumed and helps in burning that substance. Though oxygen is found beyond 100 km but it is reasonably in good proportion within 16 km of height.With increasing height, the amount of oxygen decreases very rapidly. On mountain slope, the available oxygen for breath is very scanty and the mountaineers are supposed to carry oxygen for them.
Argon
[edit | edit source]In terms of percentage, argon is the third largest gas in the atmosphere constituting 0.934 percent of total dry atmosphere . It is an inert gas and chemically it is inactive. It is also found in the earth’s crust and sea water. It is used in electric bulb and fluorescent lights.
Carbon-dioxide
[edit | edit source]It is the fourth abundant gas of the atmosphere. It is densest gas and found in lower parts. It is found upto a height of about 30 km, it is concentrated in the lower strata. Its percentage is very low, i.e., 0.04 percent but it is most vital for the growth of vegetative life of biosphere. It is transparent to the incoming solar radiation but does not allow to escape the same. And hence, it is called as greenhouse gas. It plays a very crucial role in increasing the global temperature.
Methane
[edit | edit source]Methane is the also a greenhouse gas which absorbs the radiation and cause more temperature of the air. Paddy cultivation also generates methane in the air. It is also produced from the wetlands and waterlogged soils and released in the atmosphere. Fossil fuel is also a source to release of methane in the atmosphere. Its amount in the atmosphere is variable.
Ozone
[edit | edit source]Ozone’s concentration lays in a belt between the heights of 15 to 50 km of atmosphere. Instead ofnormal two atoms of oxygen, ozone has three atoms of oxygen formed together denoted by O3. It is formed when atmospheric oxygen molecules are broken by ultraviolet solar radiation. It may even be formed at the time of electrical discharge during thunderstorms. This gas is also termed as variable as its formation and disintegration is dependent upon numerous activities. Though ozone is very less in quantity (0.00006 percent), this thin layer is very significant for the survival of living worldas it absorbsthe dangerous ultraviolet rays and protects the earth. Neon, helium, krypton, hydrogen, xenon are other minor gases. Some gases are still extremely less in quantity, they are termed as trace gases. Important among them are ammonia, carbon monoxide, sulphur dioxide, nitrogen dioxide, nitrous oxide and sulphur hexafluoride etc.
Water Vapour
[edit | edit source]Water vapour is small in amount but it is one of the most important part of atmosphere with respect to the distribution of vegetation and life. Water vapour exists all the time in the atmosphere but with varying degree of amount depending upon the season (temperature condition) and the supply of water for evaporation and evapotranspiration. Air is hardly completely dry. In summer, the water holding capacity of the air is large as the temperature is high while in winter it is low.
Aerosols
[edit | edit source]Aerosols are extremely fine-sized solid particles or liquid droplets which continue to be in suspended form in gas for very-very long time. They could be seen when their concentration is more otherwise they are invisible. Aerosols themselves are non-gaseous microscopic substance released in the atmosphere from various sources –natural and human created. They could be pollen, minute earthly dust, sea salt, carbon soot from burning fuels, volcanic dust etc. Human activities also help the aerosols to enter the atmosphere. Their concentration is more over the industrial and urban areas. Burning of fossil fuels and generation of smoke also pump the aerosols in the air.

Meaning of Structure of Atmosphere
[edit | edit source]Structure means the arrangement of different part into one. In another words, it is the skeleton or organization or anatomy of a whole by looking at the relationships with its parts. According to this background, the study of different parts of the atmosphere and the relationship with its parts is said to be the structure of the atmosphere. Vertically, the atmosphere is divided into different layers/ parts. Therefore, the study of different layers is known as structure of atmosphere.
Structure of Atmosphere
[edit | edit source]Based on chemical composition, the atmosphere is classified into two. They are homosphere and heterosphere.
Homosphere
[edit | edit source]Homosphere is that part of atmosphere where the chemical composition of the air is uniform or similar. It is the lowest layer in terms of chemical composition. It extends from the earth’s/ ocean surface to about 85 km. the basis of the changes in temperature, the atmosphere is divided into five layers out of them, three lower layers falls under homosphere (i.e. within 85 km of altitude). They are troposphere, stratosphere and mesosphere.
Troposphere
[edit | edit source]It is the lowest and densest layer of the atmosphere. It extends till a height of about 8 km over pole but over equator, it is 18 km. About 80 percent of the total mass of the atmosphere lays in this layer. With increase in height, the temperature keeps on declining till the limit of this layer. On an average, the decrease in temperature with height is 60 Celsius par km. The upper boundary is known as troposphere laying between 8 and 18 km. At this level, the average temperature reaches to minus 500 to minus 600 Celsius.
Stratosphere
[edit | edit source]Stratosphere is the upward second layer as well as middle layer of the homosphere. It starts from tropopause to approximate height of 50 km. The temperature at the tropopause remains almost constant till the height of 20 km. After that, it starts increasing and continue the trend till the height of 50 km. At this level, the estimated temperature is about minus 100 to minus 150Celsius. Though the temperature is on rise, but there is no atmospheric turbulence. This layer is completely free from clouds and other weather conditions. That is why, it has an advantage for flying long-distance supersonic jets.
Mesosphere
[edit | edit source]
Mesosphere is the third but the upper-most layer of the homosphere. After this layer, heterosphere starts. The literal meaning of mesosphere is the middle sphere. It is separated by tropopause below from troposphere and mesopause on the top from thermosphere. It is extended from 50 km to 85 km from the earth’s surface. The air pressure is very low. It is 1 millibar at the lower limit whereas it is 0.01 millibar at the highest limit. This layer is characterised by decreasing temperature and the coldest/ lowest atmospheric temperature is recorded in this layer. The lowest temperature estimated near the mesosphere is around minus 1300Celsius. It is colder that the lowest temperature recorded over Antarctic.
Heterosphere
[edit | edit source]The atmosphere laying beyond the homosphere is termed as heterosphere. The term itself is self-explanatory and it is used for that part of atmosphere where the air is not uniform. In this part of atmosphere, the air is rare and the molecules are wide apart. Relatively heavier gas molecules are concentrated in the lower part whereas the lighter are forced to be above. Beyond 85 km height, the composition of the atmosphere with increasing altitude vary significantly. Different layers of prominently different gases are nitrogen layer, oxygen layer, helium layer and hydrogen layer are differentiated. However, the heterosphere, is divided into two main spheres –thermosphere and exosphere.
Thermosphere
[edit | edit source]
This sphere extends from mesopouse. it is 85 km to about 650 km from earth. The temperature is on rise in this layer due to absorption of solar radiation by small amount of oxygen molecules present. It is highly dependent upon the solar activities. The temperature reaches beyond 12000C at an altitude of about 350 km but by 650 km it may even rise to 20000C. This much high temperature is primarily defined by average speed with which molecules are moving. Because of this, the temperature may be high.

Exosphere
[edit | edit source]Exo means external. Therefore, exosphere the external or the outer most layer of the atmosphere. Its lower boundary starts from the thermopause 650 km to 10000 km. This much distance is little less than the diameter of the earth. It is really a very big size of the limit of the atmosphere.
Lesson Summary
[edit | edit source]- Different temperature gradients create different layers within the atmosphere. The lowest layer is the troposphere, where most of the atmospheric gases and all of the planet's weather are located.
- The troposphere gets its heat from the ground, and so temperature decreases with altitude. Warm air rises and cool air sinks and so the troposphere is unstable.
- In the stratosphere, temperature increases with altitude. The stratosphere contains the ozone layer, which protects the planet from the Sun's harmful UV. The higher layers contain few gas molecules and are very cold.