Chemistry 101/Printable version
| This is the print version of Chemistry 101 You won't see this message or any elements not part of the book's content when you print or preview this page. |
The current, editable version of this book is available in Wikibooks, the open-content textbooks collection, at
https://en.wikibooks.org/wiki/Chemistry_101
Elements and Atoms: The Basics of Chemistry
Chapter 1: Elements and Atoms: The Basics of Chemistry
[edit | edit source]- Writing Convention/How to read this book
- The Importance of Chemistry
- The Scientific Method: Observing Nature
- How to Classify Materials by Properties
- How to Describe Materials by Properties
- Atoms of an Element
- Introducing Sub-atomic Particles
- Purpose of The Periodic Table
Elements and Atoms: The Basics of Chemistry/Conventions
Writing Conventions/How to read this Book
[edit | edit source]Book Organization
[edit | edit source]This book is divided into Chapters, which are further divided into sections. The section pages are those which contain the content for reading. The book page contains the complete table of contents, while each chapter page contains the table of contents only for that chapter. Given this explanation, navigation should be self explanatory using the small links in the top left. However, additional navigation capabilities will be added in the future to make things even easier.
Definition of key terms
[edit | edit source]When key terms appear in the text they are denoted in one of two ways.
- If the key term is defined within the text, directly before or after the term appears, then it is shown as italicized text.
- If the term is left to be formally defined at the end of the section(bottom of page), then it will be shown in boldface text.
1.2 -- The Importance of Chemistry
Elements and Atoms: The Basics of Chemistry/The Importance of Chemistry
Section 1: The Importance of Chemistry
[edit | edit source]So many of the luxuries we have become accustomed to today would not have been possible without recent advancements in the physical sciences, namely Chemistry. One must think of all the super strong adhesives, fibers, and building materials that are used everyday in construction, and production. Without an understanding of Chemistry, the development of these materials, in addition to the development of things like prescription drugs, would never have been possible. And while these advents are useful and interesting to the layman, an understanding of Chemistry is even more vital for an aspiring scientist. The applications that chemistry has to all scientific disciplines are enumerable. For example, it is an understanding of the behavior of matter on a small scale that allows chemical engineers to design and produce the interesting systems that run our world's infrastructure.
Chemistry, as a science, studies the composition and properties of matter. Chemistry also studies the reactions between different chemicals, known as chemical reactions. Basic chemical reactions are always going on around us as well as a more complex system of reactions, even just within our bodies. For example, as anyone sits down at his computer and begins to type, his body undergoes a huge amount of chemical reactions to make his eyes and hands move, and to make his brain think. This is an example of the Chemistry of biological processes. Some chemists study these biological processes, while others may study different things. In general, all chemists do the following:
- Chemists try to understand how the composition of substances affect the substance's properties.
- Chemists seek to understand why and how substances change within chemical reactions.
- Chemists try to understand and observe the underlying structure of matter.
- Chemists attempt to understand why the properties that we observe with our senses occur in matter.
Of course, taking a first class in Chemistry does not mean you are going to become a chemist. However, Chemistry is useful in all branches of science. Even for a non science major, chemistry can improve one's understanding of the world around them, and in my experience, can help you win some arguments. Chemistry is used in Physics, Biology, Engineering, Electronics, Pharmaceuticals etc. So you know what chemists do, in a very general sense. So you may be wondering why chemistry is so important to all of the sciences. The answer is that all things are composed of chemicals. This includes your hair, your water, your computer, and your dinner. Even living things are composed entirely of chemicals. You may already know this, and say "duhh", but think of the implications this has. Everything you know is made of matter, and contains chemicals that are able to participate in complex reactions. You can see why Chemistry is such a deep field of study. So, while scientific disciplines may have varying interests, they all use chemistry, and today the lines between Physics, Biology, and Chemistry are more hazy than ever.
- Matter
- Matter can be defined as anything that has Mass and also takes up space (has volume).
- Mass
- Mass is a measure of the amount of matter an object contains. Mass is often thought to be the same as weight, but it is not. Weight refers to the force of which an object of a particular mass is attracted by gravitational forces. We will discuss mass more formally later. An easy way to understand this concept is that weight is gravity dependent, while mass is gravity independent.
- Volume
- Volume can be thought of as the amount of 3-dimensional space occupied by an object.
Elements and Atoms: The Basics of Chemistry/The Scientific Method: Observing Nature
The Method and Definition of Observation
[edit | edit source]Drawing Conclusions based on Observations
[edit | edit source]Conclusions Can be Revised
[edit | edit source]Conclusions can, and should be revised. A conclusion can change based on new evidence and observations. However, an observation should never change based on new evidence or further observation. An observation, by definition, should only describe the physical phenomenon occurring in a particular situation. Again, an observation describes what is happening only as it is seen.
Process of the Scientific Method
[edit | edit source]1.) Ask a question
2.) Do background research
3.) Construct a hypothesis
4.) Conduct an experiment to test your hypothesis
5.) Analyze the data and draw a conclusion
Elements and Atoms: The Basics of Chemistry/Atoms of an Element
An atom is an element that takes part in a chemical reaction. Atoms have a nucleus.
Elements and Atoms: The Basics of Chemistry/Introducing Sub-atomic Particles
More than hundred particles are present with an atom e.g. neutron, proton and electron etc.
| This page or section is an undeveloped draft or outline. You can help to develop the work, or you can ask for assistance in the project room. |
Elements and Atoms: The Basics of Chemistry/Purpose of The Periodic Table
History of The Periodic Table(s)
[edit | edit source]One of the original periodic tables was a creation of Dmitri Mendeleev, a Russian scientist.
After becoming a teacher, Mendeleev wrote a definitive two-volume textbook at that time: Principles of Chemistry (1868–1870). As he attempted to classify the elements according to their chemical properties, he noticed patterns that led him to postulate his Periodic Table. Mendeleev was unaware of the other work on periodic tables going on in the 1860s. He first made a small table following a particular pattern, and by adding additional elements following this pattern, developed his extended version of the periodic table, pictured here.[1]
As you can see the table consists of rows, and columns. In the case of a periodic table, the rows are referred to as periods, and the columns are referred to as groups. This table is very similar to the modern periodic table. In the modern periodic table there are seven periods, and eighteen groups. The labeling of the groups in the table can vary from country to country. You will see more on this soon.
Purpose of The Periodic Table
[edit | edit source]Divisions of The Periodic Table
[edit | edit source]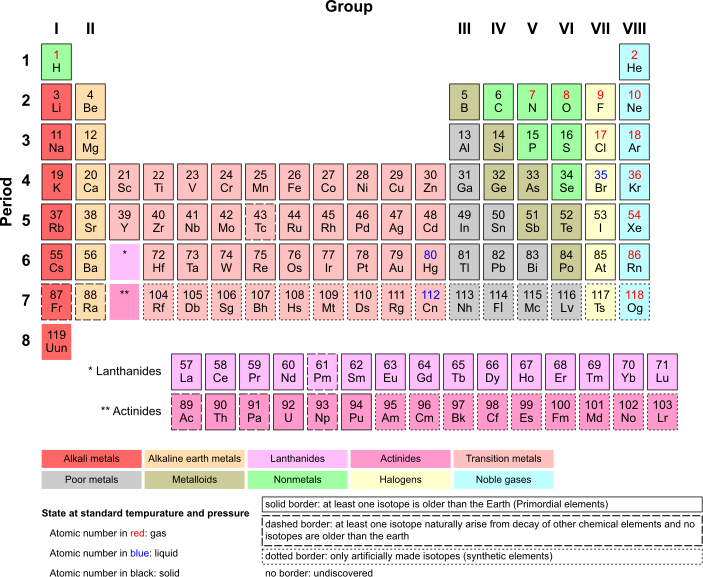 Periodic Table Roman numerals for A groups...
Periodic Table Roman numerals for A groups...
How Chemists use the Periodic Table
[edit | edit source]Atomic numbers and subatomic particles
[edit | edit source]References
[edit | edit source]- ↑ Dmitri Mendeleev(Wikipedia), Wikipedia.org

