Chemical Sciences: A Manual for CSIR-UGC National Eligibility Test for Lectureship and JRF/Named Reactions/Beckmann Rearrangement
| This page was imported and needs to be de-wikified. Books should use wikilinks rather sparsely, and only to reference technical or esoteric terms that are critical to understanding the content. Most if not all wikilinks should simply be removed. Please remove {{dewikify}} after the page is dewikified. |
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is an acid-catalyzed rearrangement of an oxime to an amide.[1][2][3] Cyclic oximes yield lactams.

This example reaction[4] starting with cyclohexanone and forming caprolactam is one of the most important applications of the Beckmann rearrangement, as caprolactam is the feedstock in the production of Nylon 6.
The Beckmann solution consists of acetic acid, hydrochloric acid and acetic anhydride, and was widely used to catalyze the rearrangement. Other acids, such as sulfuric acid or polyphosphoric acid, can also be used. sulfuric acid is the most commonly used acid for commercial lactam production due to its formation of an ammonium sulfate by-product when neutralized with ammonia. Ammonium sulfate is a common agricultural fertilizer providing nitrogen and sulfur.
Reaction mechanism
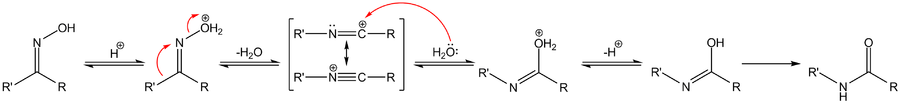
[edit | edit source]The reaction mechanism of the Beckmann rearrangement is generally believed to consist of an alkyl migration with expulsion of the hydroxyl group to form a nitrilium ion followed by hydrolysis:
In one study, [5] the mechanism is established in silico taking into account the presence of solvent molecules and substituents. The rearrangement of acetone oxime in the Beckmann solution involves three acetic acid molecules and one proton (present as an oxonium ion). In the transition state leading to the iminium ion (σ - complex), the methyl group migrates to the nitrogen atom in a concerted reaction and the hydroxyl group is expulsed. The oxygen atom in the hydroxyl group is stabilized by the three acetic acid molecules. In the next step the electrophilic carbon atom in the nitrilium ion is attacked by water and the proton is donated back to acetic acid. In the transition state leading to the N-methyl acetimidic acid, the water oxygen atom is coordinated to 4 other atoms. In the third step, an isomerization step protonates the nitrogen atom leading to the amide.

The same computation with a hydroxonium ion and 6 molecules of water has the same result but when the migrating substituent is phenyl in the reaction of acetophenone oxime with protonated acetic acid the mechanism favors the formation of an intermediate three-membered π - complex. This π - complex is again not found in the H3O+(H2O)6.

With the cyclohexanone-oxime, the relief of ring strain results in a third reaction mechanism leading directly to the protonated caprolactam in a single concerted step without the intermediate formation of a π - complex or σ - complex.
Cyanuric chloride assisted Beckmann reaction
[edit | edit source]The Beckmann reaction is known to be catalyzed by cyanuric chloride and zinc chloride co-catalyst. For example, cyclododecanone can be smoothly converged to the corresponding lactam, a monomer for the production of nylon 12.[6]

The reaction mechanism for this reaction is based on a catalytic cycle with cyanuric chloride activating the hydroxyl group via a nucleophilic aromatic substitution. The reaction product is dislodged and replaced by new reactant via an intermediate Meisenheimer complex.

Beckmann fragmentation
[edit | edit source]When the oxime has a quaternary carbon atom in an anti position to the hydroxyl group a fragmentation occurs forming a nitrile:
The fluorine donor in this fragmentation reaction is DAST [7]:
Semmler-Wolff reaction
[edit | edit source]The oxime of cyclohexenone with acid forms aniline in a dehydration - aromatization reaction called the Semmler-Wolff reaction or Wolff aromatization [8] [9] [10] [11]
References
[edit | edit source]- ↑ Beckmann, E. (1886). "Zur Kenntniss der Isonitrosoverbindungen". Berichte der Deutschen Chemischen Gesellschaft. 19: 988–993. doi:10.1002/cber.188601901222.
- ↑ Donaruma, L. G. (1960). "The Beckmann rearrangement. (Review)". Org. React. 11: 1–156.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ Gawley, R. E. (1988). "The Beckmann reactions: rearrangement, elimination-additions, fragmentations, and rearrangement-cyclizations. (Review)". Org. React. 35: 14–24.
- ↑
Eck, J. C. (1939). "Ε-Benzoylaminocaproic Acid". Organic Syntheses, Coll. 19: 20.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Eck, J. C. (1943). "Ε-Benzoylaminocaproic Acid". Organic Syntheses, Coll. 2: 76.{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ Yamabe, S. (2005). "Is the Beckmann Rearrangement a Concerted or Stepwise Reaction? A Computational Study". Journal of Organic Chemistry. 70: 10638–10644. doi:10.1021/jo0508346.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ Furuya, Y. (2005). "Cyanuric Chloride as a Mild and Active Beckmann Rearrangement Catalyst". Journal of the American Chemical Society. 127: 11240–11241. doi:10.1021/ja053441x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ Kirihara, Masayuki (1997). "Fluorinative -cleavage of cyclic ketoximes with diethylaminosulfur trifluoride: an efficient synthesis of fluorinated carbonitriles". Chemical Communications. 6: 599–600. doi:10.1039/a607749h.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ W. Semmler, Ber. 25, 3352 (1892)
- ↑ L. Wolff, Amp. 322, 351 (1902)
- ↑ Name reactions and reagents in organic synthesis, Bradford P. Mundy,Michael G. Ellerd,Frank G. Favaloro
- ↑ Beckmann Rearrangements. An Investigation of Special Cases E. C. Horning, V. L. Stromberg, H. A. Lloyd J. Am. Chem. Soc., 1952, 74 (20), pp 5153–5155 Template:DOI