Applied Science BTEC Nationals/Scientific Practical Techniques
Content
[edit | edit source]On completion of this unit a learner should:
- 1. Be able to use analytical techniques
- 2. Be able to use scientific techniques to separate and assess purity of substances
- 3. Be able to use instruments/sensors for scientific investigations.
Chromatography
[edit | edit source]Colorimetry
[edit | edit source]Colorimetry is "the science and technology used to quantify and describe physically the human color perception." It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color perception, most often the CIE 1931 XYZ color space tristimulus values and related quantities.
Titration
[edit | edit source]In medicine, titration is the process of gradually adjusting the dose of a medication until the desired effect is achieved.
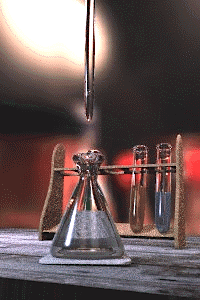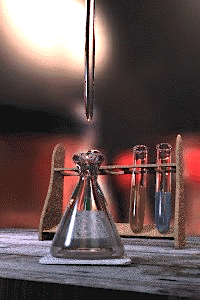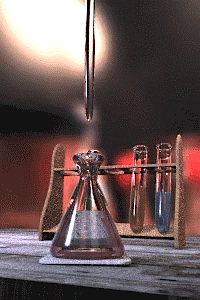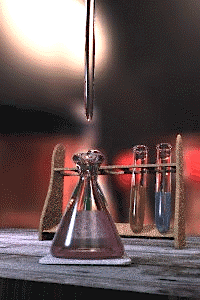
Titration is a common laboratory method of quantitative/chemical analysis which can be used to determine the w:concentration of a known w:reactant. Because volume measurements play a key role in titration, it is also known as volumetric analysis.
A w:reagent, called the titrant, of known concentration (a w:standard solution) and w:volume is used to react with a solution of the w:analyte, whose concentration is not known in advance. Using a calibrated w:burette to add the titrant, it is possible to determine the exact volume that has been consumed when the endpoint is reached. The endpoint is the point at which the titration is complete, often determined by an indicator (see below). In the classic strong acid-strong base titration the endpoint of a titration is when the pH of the reactant is just about equal to 7, and often when the solution permanently changes colour due to an indicator. There are however many different types of titrations (see below).
Many methods can be used to indicate the endpoint of a reaction; titrations often use visual indicators (the reactant mixture changes colour). In simple w:acid-base titrations a pH indicator may be used, such as w:phenolphthalein, which becomes pink when a certain pH (about 8.2) is reached or exceeded. Another example is w:methyl orange, which is red in acids and yellow in alkali solutions.
Not every titration requires an indicator. In some cases, either the reactants or the products are strongly coloured and can serve as the "indicator". For example, an oxidation-reduction titration using w:potassium permanganate (pink/purple) as the titrant does not require an indicator. When the titrant is reduced, it turns colourless. After the equivalence point, there is excess titrant present. The equivalence point is identified from the first faint pink colour that persists in the solution being titrated.
Due to the logarithmic nature of the pH curve, the transitions are generally extremely sharp, and thus a single drop of titrant just before the endpoint can change the pH significantly — leading to an immediate colour change. That said, there is a slight difference between the change in indicator colour and the actual equivalence point of the titration. This error is referred to as an indicator error, and it is indeterminate.
Preparing a sample for titration
[edit | edit source]In a titration, both titrant and analyte are required to be aqueous, or in a solution form. If the sample is not a liquid or solution, the samples must be dissolved. If the analyte is very concentrated in the sample, it might be useful to dilute the sample.
Although the vast majority of titrations are carried out in aqueous solution, other solvents such as glacial ethanoic acid or ethanol (in petrochemistry) are used for special purposes.
A measured mass of the sample can be placed in a flask and then be dissolved or diluted. The mathematical result of the titration can be calculated directly. Sometimes the sample is dissolved or diluted beforehand and a measured volume of the solution is used for titration. In this case the dissolving or diluting must be done accurately because the mathematical result of the titration must be multiplied with this factor.
Some non-acid-base titrations require buffering to maintain a certain pH for the reaction. Therefore, buffer solutions are added to the reactant solution in the flask.
Some titrations require "masking" of a certain ion. This can be necessary when two reactants in the sample would react with the titrant and only one of them must be analysed, or when the reaction would be disturbed or inhibited by this ion. In this case another solution is added to the sample which "masks" the unwanted ion (for instance by a weak binding with it or even forming a solid insoluble substance with it).
Some reactions may require heating the solution with the sample and titration while the solution is still hot (to increase the reaction rate).
Procedure
[edit | edit source]A typical titration begins with a conical flask containing a precisely known volume of the reactant and a small amount of indicator, placed underneath a w:burette containing the reagent. By controlling the amount of reagent that is added to the reactant, it is possible to detect the point at which the indicator changes colour. As long as the indicator has been chosen correctly, this should also be the point where the reactant and reagent neutralise each other, and by reading the scale on the burette the volume of reagent can be measured.
As the concentration of the reagent is known, the number of moles of reagent can be calculated (since ). Then, from the chemical equation involving the two substances, the number of moles present in the reactant can be found. Finally, by dividing the number of moles of reactant by its volume, the concentration is calculated.
Types of titrations
[edit | edit source]Titrations can be classified by the type of reaction. Different types of titration reaction include:
- w:Acid-base titration is based on the neutralisation reaction between the analyte and an acidic or basic titrant. These most commonly use a pH indicator, a pH meter, or a conductance meter to determine the endpoint.
- A w:Redox titration is based on an oxidation-reduction reaction between the analyte and titrant. These most commonly use a potentiometer or a redox indicator to determine the endpoint. Frequently either the reactants or the titrant have a colour intense enough that an additional indicator is not needed.
- A w:Complexometric titration is based on the formation of a complex between the analyte and the titrant. The w:chelating agent w:EDTA is very commonly used to titrate metal ions in solution. These titrations generally require specialised indicators that form weaker complexes with the analyte. A common example is w:Eriochrome Black T for the titration of w:calcium and w:magnesium ions.
- A form of titration can also be used to determine the concentration of a w:virus or w:bacterium. The original sample is diluted (in some fixed ratio, such as 1:1, 1:2, 1:4, 1:8, etc.) until the last dilution does not give a positive test for the presence of the virus. This value, the w:titre, may be based on w:TCID50, w:EID50, w:ELD50, LD50 or pfu. This procedure is more commonly known as an w:assay.
Measuring the endpoint of a titration
[edit | edit source]Different methods to determine the endpoint include:
- w:pH indicator: This is a substance that changes colour in response to a chemical change. An acid-base indicator (e.g., w:phenolphthalein) changes colour depending on the w:pH. Redox indicators are also frequently used. A drop of indicator solution is added to the titration at the start; when the colour changes the endpoint has been reached.
- A w:potentiometer can also be used. This is an instrument which measures the w:electrode potential of the solution. These are used for titrations based on a redox reaction; the potential of the working electrode will suddenly change as the endpoint is reached.
- w:pH meter: This is a potentiometer which uses an electrode whose potential depends on the amount of H+ ion present in the solution. (This is an example of an w:ion selective electrode. This allows the pH of the solution to be measured throughout the titration. At the end point there will be a sudden change in the measured pH. It can be more accurate than the indicator method, and is very easily automated.
- Conductance: The conductivity of a solution depends on the ions that are present in it. During many titrations, the conductivity changes significantly. (For instance, during an acid-base titration, the H+ and OH- ions react to form neutral H2O. This changes the conductivity of the solution.) The total conductance of the solution depends also on the other ions present in the solution (such as counter ions). Not all ions contribute equally to the conductivity; this also depends on the mobility of each ion and on the total concentration of ions (w:ionic strength). Thus, predicting the change in conductivity is harder than measuring it.
- Colour change: In some reactions, the solution changes colour without any added indicator. This is often seen in redox titrations, for instance, when the different oxidation states of the product and reactant produce different colours. Such titrations are known as 'self-indicating'.
- Precipitation: If the reaction forms a solid, then a w:precipitate will form during the titration. A classic example is the reaction between Ag+ and Cl- to form the very insoluble salt AgCl. Surprisingly, this usually makes it difficult to determine the endpoint precisely. As a result, precipitation titrations often have to be done as "back" titrations (see below).
- An isothermal titration calorimeter uses the heat produced or consumed by the reaction to determine the endpoint. This is important in biochemical titrations, such as the determination of how substrates bind to w:enzymes.
- Thermometric titrimetry is an extraordinarily versatile technique. This is differentiated from calorimetric titrimetry by the fact that the heat of the reaction (as indicated by temperature rise or fall) is not used to determine the amount of analyte in the sample solution. Instead, the endpoint is determined by the rate of temperature change.
- w:Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the w:spectrum of the reactant, titrant or product is known. The relative amounts of the product and reactant can be used to determine the endpoint.
- w:Amperometry can be used as a detection technique (w:amperometric titration). The current due to the oxidation or reduction of either the reactants or products at a working electrode will depend on the concentration of that species in solution. The endpoint can then be detected as a change in the current. This method is most useful when the excess titrant can be reduced, as in the titration of halides with Ag+. (This is handy also in that it ignores precipitates.)
Other terms
[edit | edit source]The term w:back titration is used when a titration is done "backwards": instead of titrating the original analyte, one adds a known excess of a standard reagent to the solution, then titrates the excess. A back titration is useful if the end point of the reverse titration is easier to identify than the end point of the normal titration. They are also useful if the reaction between the analyte and the titrant is very slow.
Particular uses
[edit | edit source]- As applied to w:biodiesel, titration is the act of determining the w:acidity of a sample of WVO by the dropwise addition of a known base to the sample while testing with w:pH paper for the desired neutral pH=7 reading. By knowing how much base neutralizes an amount of WVO, we discern how much base to add to the entire batch.
- Titrations in the w:petrochemical or food industry to define oils, fats or biodiesel and similar substances. An example procedure for all three can be found here: [1].
- w:Acid number: an acid-base titration with colour indicator is used to determine the free fatty acid content. See also: pH of fatty acids.
- w:Iodine number: a redox titration with colour indication which indicates the amount of unsaturated fatty acids.
- w:Saponification value: an acid-base back titration with colour indicator or potentiometric to get a hint about the average chain length of fatty acids in a fat.
Sources
[edit | edit source]Adapted from w:Titration
Sampling
[edit | edit source]The importance of representative sampling
[edit | edit source]“The fundamental principle behind any sampling activity is that a small amount of collected material should be representative of all the material being monitored. The number and location of samples that are needed to make up a representative sample depends on how homogeneous the material is. If it is very homogeneous, only a few samples may be required. If the material is heterogeneous, many more samples will be required.”[1]
Monitoring can be classified into types
[edit | edit source]- Periodic measurements – measurements are made at periodic intervals; for example, once every three months. The sample is often withdrawn from the sampling site (extractive sampling). An instrumental or automated technique may be used, where the sampling and analysis of the substance is fed to an on-line analyser. Alternatively, a technique may be used where a sample is extracted on site and analysed later in a laboratory. Samples may be obtained over several hours, or may be so-called “spot” or “grab” samples collected over a period of seconds to several minutes.[1]
- Intermittent measurements are made when convenient/possible. The timing between measurements may be semi-periodic (e.g. every day, sometime during working hours).
- Continuous emissions monitoring systems (CEMs) are automated measurements carried out continuously, with few (if any) gaps in the data produced. Measurement may be carried out in situ, or extractive sampling may be used with an instrument permanently located at or near the stack. CEMs are also referred to as Automated Monitoring Systems (AMS), particularly in Europe.[1]
Should ambient air sampling be continuous or intermittent?
[edit | edit source]Consider:[2]
- the averaging period of the relevant quality criteria with which the data will be compared
- whether the impact is acute or chronic
- the detail required, e.g. short peaks averaged over three minutes, one-hour averages, daily averages, etc.
Short sampling programmes are unlikely to give data representative of long-term conditions. Meteorological conditions and source variations can have significant effects on pollutant concentrations. Where short-term peaks are of interest, these may be unusual events occurring for only a few days each year. Hence short-term monitoring campaigns are of very limited value for characterising air-pollution episodes.[2]
Comparison of continuous and periodic monitoring for water analysis
[edit | edit source]| Characteristic | Continuous water monitoring systems (CWMs) | Periodic monitoring |
| Sampling period | Monitoring covers all or most of the period that substances are discharged | Snapshots of the long-term discharge profile |
| Speed of results generation | Almost always real-time output of results | Real-time results if portable instrumental analysers used; delayed results if laboratory end-method used |
| Stability | Sensors may be prone to fouling or other malfunction | Sample integrity needs to be maintained before analysis |
| Availability | Only a limited number of methods possible | Comprehensive range of methods available |
| Applicability | May not be able to meet performance requirements at present | Methods that will meet the performance of most regulatory requirements are available |
| Reporting of results | Results continuously averaged over (typically) one hour or 24 hours | Results reported as daily average or instantaneous |
| Capital cost | Tends to be higher than equivalent periodic monitoring method | Tends to be lower than equivalent CWMs |
Definitions of important terms
[edit | edit source]Sampling plane – the plane at 90° to the centreline of the stack, duct or channel at the sampling location.
Sampling point – the specific position on the sample plane from where the sample is extracted.
Isokinetic sampling – when the flow enters the sampling nozzle at the same velocity and direction as usual flow at the sampling point.
Representative sampling of particulates, gases and liquids
[edit | edit source]Although a flowing gas or liquid might be thought of as being more homogeneous than, for example, a stockpile of coal; such flows can become heterogeneous. This may be due to differences in chemical composition, or differences in temperature and velocity, which may lead to stratification and swirling. Where the flow is also carrying particulates or aerosols along the duct or channel, there is likely to be even less uniformity. Here, special measures must be taken to ensure samples are representative.
If the flowing sample may be heterogeneous, samples must be obtained from multiple sample points across the sampling plane to give an overall average value.
If the emission rate is to be calculated, the flowrate will need to be measured; this will require velocity measurements to be made at several points across the sampling plane.
For extractive methods involving gases or liquids carrying particulates or aerosols, the sample must be collected isokinetically.
Ambient air sampling
[edit | edit source]Where to sample?
[edit | edit source]Spatial considerations encompass both the location of monitoring positions relative to study area or emission source, and individual sampling site criteria, e.g. position relative to local emission sources and any interfering effects.
Data handling and data analysis.
[edit | edit source]Is the speed of results generation important? For example, results may be required in real time for public health warnings, whereas several weeks turnaround may be adequate for supplying routine results for authorisation compliance monitoring.
How to sample?
[edit | edit source]Both the type of sampling and analytical end method need to be considered. Sampling may be unidirectional or omnidirectional; in-situ, mobile or remote sensing. Method selection involves an appraisal of cost versus performance, the latter including limits of detection, sensitivity, speed of instrument response, susceptibility to interfering species and the overall uncertainty of the measurement.
Supplementary data collection.
[edit | edit source]Other information may be relevant to the study, for example meteorological conditions, process data and traffic flows. Meteorological conditions are obviously important in assessing the impact of a single source on its surroundings since they dictate the transport and dispersion of pollutants in ambient air. Many chemical transformations between reactive species in the atmosphere are also influenced.
Soil sampling
[edit | edit source]If a property of the sample changes over time, then the analysis shall be carried out without as soon as possible.
If a sample has been stabilised, or preserved then this fact shall be recorded when the results are reported and details of the stabilising procedure or preserving agent shall be recorded.
When a sample has been dried and is subsequently analysed, sufficient information shall be provided to establish the stability of the parameter analysed. Such information shall provide justification for analysing the dried sample, rather than analysing the sample on a “wet-weight” or “as submitted” basis.
References
[edit | edit source]Resources
[edit | edit source]Edexcel recommend the following resources except * which have been added to their list.
External links
[edit | edit source]- Science aid: Titration An informative yet simple explanation of titration aimed at teens
- Titration - apparatus, technique and calculation
- Titration freeware - simulation of any pH vs. volume curve, distribution diagrams and real data analysis
- Free Metrohm Monograph: Practical Titration
- Free Metrohm Monograph: Practical Thermometric Titrimetry
- Basics of Titration: Free downloadable guide (pdf) from METTLER TOLEDO
Textbooks
[edit | edit source]Coyne G S — The Laboratory Companion: A Practical Guide to Materials, Equipment and Technique (John Wiley & Sons, 2005) ISBN 0471780863
Dean J R et al. — Practical Skills in Chemistry (Prentice Hall, 2001) ISBN 013028002X
Dean J R et al. — Practical Skills in Forensic Science (Prentice Hall, 2005) ISBN 0131144006
Derenzo S E — Practical Interfacing in the Laboratory: Using a PC for Instrumentation, Data Analysis and Control (Cambridge University Press, 2003) ISBN 0521815274
Jones A et al. — Practical Skills in Biology, 3rd Edition (Prentice Hall, 2002) ISBN 013045141X
Lintern M — Laboratory Skills for Science and Medicine: An Introduction (Radcliffe Medical Press, 2006) ISBN 1846190169
Prichard E and Lawn R — Practical Laboratory Skills Training Guide: Measurement of pH (The Royal Society of Chemistry, 2003) ISBN 0854044736
Prichard E and Lawn R — Practical Laboratory Skills Training Guide: Measurement of Volume (The Royal Society of Chemistry, 2003) ISBN 085404468X
Reed R et al. — Practical Skills in Biomolecular Science (Prentice Hall, 2003) ISBN 0130451428
Journals
[edit | edit source]Nature
New Scientist
