Analytical Chemiluminescence/Printable version
| This is the print version of Analytical Chemiluminescence You won't see this message or any elements not part of the book's content when you print or preview this page. |
The current, editable version of this book is available in Wikibooks, the open-content textbooks collection, at
https://en.wikibooks.org/wiki/Analytical_Chemiluminescence
Electronic transitions and luminescence
A. Introduction
[edit | edit source]A1. Electronic transitions and luminescence
[edit | edit source]Luminescence is the emission of light due to transitions of electrons from molecular orbitals of higher energy to those of lower energy, usually the ground state or the lowest unoccupied molecular orbitals. Such transitions are referred to as relaxations. Figure A1.1 shows four electronic energy levels (S0,S1, S2 and T1) and the possible transitions between them. S0 represents the ground state, while S1, S2 and T1 represent higher-energy excited states;S0, S1 and S2 are singlet states in which all the electrons form pairs of opposed spins whereas T1 is a triplet excited state, in which not all electrons are paired off in this way.

Figure A1.1 – Jablonski diagram showing four electronic energy levels S0, S1, S2 and T1, with their vibrational fine structure and the transitions between them that affect luminescence.
Each energy level is subdivided into a number of vibrational states, each characterised by an amount of vibrational energy that accompanies the potential energy of the electrons occupying the orbitals. Luminescence is classified according to the excited state that gives rise to it and to the source of the energy that caused the excited state to be populated with electrons. The promotion of electrons to an excited state is called excitation. In many cases, this is brought about by absorption of visible or ultraviolet radiation. In such a case, if the luminescence arises because electrons are relaxing from a singlet excited state to a singlet ground state, then it is called fluorescence, and generally occurs within 10−11 to 10−5 s. The transition is very fast because it involves no reversal of electron spin. If, however, it arises due to relaxation from a triplet excited state, then the luminescence is called phosphorescence, which generally occurs within 10−4 to 100 s. If the excitation is the result of energy released in a chemical reaction, the luminescence is called chemiluminescence. A subset of chemiluminescence occurring in the biosphere as a result of biological processes is called bioluminescence. Electrochemiluminescence is another distinct subset of chemiluminescence phenomena, made up of those reactions in which the excited species is produced at an electrode during electrolysis.
Before luminescence occurs, there is a non-radiative loss of energy (due to collisions between molecules) as the excited state relaxes to a lower vibrational state while remaining at the same electronic energy level. This type of transition is called vibrational deactivation. It has to occur even more rapidly than fluorescence and typically occurs within 10−12 s of excitation. Therefore the luminescence involves the emission of photons of lower energy (higher wavelength) than would otherwise be the case. Another possible transition is internal conversion, in which an electron transfers from a lower vibrational state of a higher electronic energy level to a higher vibrational state of a lower electronic energy level, without any significant gain or loss of energy; such a transition, S2 → S1, is shown schematically in Figure 1.1. In intersystem crossing, internal conversion would involve also reversal of the spin of the electron as in a transition from a singlet to a triplet state; the transition S2 → T1 in Figure 1.1 is of this type. Such transitions can give rise to phosphorescence. Finally, luminescence is not inevitable. The intensity of the emission compared with the number of molecules in the excited state is called the quantum yield(ΦF). This can be calculated for fluorescent emission by dividing the number of emitted photons by the number of absorbed photons. ΦF in a chemiluminescence phenomenon should be the same as in the fluorescence phenomenon involving the same excited state, but, because chemiluminescence does not depend on the absorption of photons, it can be calculated in the same way only by performing a separate fluorescence experiment. The intensity of chemiluminescence emission is more meaningfully compared with the number of reactant molecules; this measure is called the chemiluminescence quantum yield (ΦCL). It is related to ΦF by the equation:
ΦCL = ΦC.ΦE.ΦF
where ΦC is the proportion of reactant molecules converted into product and ΦE is the proportion of product molecules formed in the excited state. ΦCL has values from 0 to 1 and reaches 0.88 for firefly luciferin in vitro.[1]
Because ΦCL depends on ΦF it would be reasonable to suppose that chemiluminescence is affected by substitution in product molecules in the same way as is fluorescence. In that case, ΦCL would be increased by electron donors and decreased by electron acceptors. There would also be an increase in ΦCL (and a bathochromic shift in emission wavelength) due to conjugated systems and in rigidly planar molecules having facilitated π-bond delocalisation. Such generalisations must be used with great care, for the only product species to which they can apply are the molecules that are actually emitting; it is by no means obvious what these are in any particular case.
References
[edit | edit source]- ↑ Seliger H H and McElroy W D, Pathways of energy transfer in bioluminescence, Radiation Res., Suppl. 2 (1960), 528-38.
Chemiluminescence spectroscopy
A2. Chemiluminescence spectroscopy
[edit | edit source]The wavelengths of chemiluminescence emission that are analytically useful depend on the characteristics of the detector. Visible emission (though it is seldom visible to the naked eye) has a wavelength range of about 400-750 nm, corresponding to enthalpy changes of exothermic reactions of between 180 and 300 kJ mol−1, provided that there is a pathway to an excited state that relaxes with the loss of a photon (see Figure 1.1). Emission intensity is proportional to the concentration of the emitting species, which is either an intermediate or a product in an electronically excited state. This concentration depends on the rate of the reaction producing it. Analytical detection of chemiluminescence usually involves no wavelength selection, i.e., it is emission photometry rather than emission spectrophotometry. Selectivity is achieved by on-line treatments rather than by processing of the signal, which has little fine-structure.[1]
Because of this, the importance of chemiluminescence spectroscopy lies more in elucidating the mechanisms of chemiluminescence reactions rather than in analytical applications. In particular, spectroscopic investigations have been found useful for the identification of the emitter species in particular chemiluminescence reactions. Thus, experimental evidence has shown that manganese(II) ion is a common emitter in chemiluminescence arising due to the reduction either of permanganate or of other higher oxidation states of manganese.[2] Using a variety of reductants, chemiluminescence spectra, corrected for wavelength-related differences in detector sensitivity, showed maximum emission at 689 nm (in hexametaphosphate) and 734 nm, (in phosphate/orthophosphoric acid) which corresponds to the emission maxima of manganese(II) phosphorescence, and is clearly distinguishable from the intense emission at 634 nm and 703 nm from singlet oxygen, which had been earlier proposed as the emitter. Diagnosis of the emitter usually cannot be based on spectroscopic evidence alone, but must make use of chemical evidence also. Identifying the emitting species in luminol chemiluminescence in aqueous solutions is an example of such an investigation. The product of the oxidation of luminol is 3-aminophthalate. The maximum emission wavelength in these conditions is 424 nm, which corresponds to the maximum wavelength of fluorescence emission from the 3-aminophthalate dianion and this species was originally accepted as the emitter. However, chemical evidence suggests that emission is from the monoanion, which has a fluorescence maximum of 451 nm. Closer examination of the chemiluminescence reaction[3] suggests that the emitter is one particular conformer of the 3-aminophthalate monoanion that has a maximum emission wavelength resembling that of the dianion.
References
[edit | edit source]- ↑ Robards K and Worsfold P J, Analytical applications of liquid phase chemiluminescence, Anal. Chim. Acta, 266 (1992), 147.
- ↑ Barnett N W, Hindson B J, Jones P and Smith T A, Chemically induced phosphorescence from manganese(II) during the oxidation of various compounds by manganese(III), (IV) and (VII) in acidic aqueous solutions, Anal. Chim. Acta, 451 (2002), 181-188.
- ↑ Lind J S, Merényi G and Eriksen T E, Chemiluminescence Mechanism of Cyclic Hydrazides Such as Luminol in Aqueous Solutions, J. Am. Chem. Soc., 105 (1983), 7655.
Luminol
B. Reagents
[edit | edit source]B1. Luminol
[edit | edit source]B1a. Introduction
[edit | edit source]Luminol is the common name for 5-amino-2,3-dihydro-1,4-phthalazinedione (often called 3-aminopthalhydrazide). Oxidation of luminol produces excited 3-aminophthalate, which on relaxation emits light (λmax = 425 nm) with quantum yield of ~0.01;[1] Information on the hazards of using luminol is available at the website of the United States National Toxicology Program [1]. The reaction is triggered by a catalytic process, usually enzymatic, provided, for example, by heme-containing proteins, especially horseradish peroxidase (HRP, EC 1.11.1.7). In the presence of hydrogen peroxide this enzyme is converted into intermediary complexes before being regenerated. It has the distinct advantage in biological work of permitting the luminol reaction at pH as low as 8.0 to 8.5. HRP can be used as a label to detect analytes of interest and luminol chemiluminescence can be used to detect substrates of oxidase enzymes that generate hydrogen peroxide. Enzymatic catalysis is discussed fully in section B1f (ADD LINK). The catalyst may be chemical rather than enzymatic (e.g., transition metal cations or complex ions, e.g., ferricyanide, at high pH). Catalysis by metal ions is discussed fully in section B1e (ADD LINK).
Alternatively, luminol chemiluminescence may be triggered electrochemically. Sakura[2] had proposed that luminol was oxidized at the electrode surface, after which it can react with hydrogen peroxide producing one photon per hydrogen peroxide molecule (compared with 0.5 in the HRP-catalysed reaction) giving more sensitive detection and avoiding the fragility of enzyme methods.[3] Luminol electrochemiluminescence is discussed fully in section B1d (ADD LINK).
Very many assays have been devised determining compounds by inhibiting, enhancing or catalysing luminol chemiluminescence. Detectivity reaches the sub-femtomole level but the very versatility of the chemistry limits its selectivity. This is a serious shortcoming for samples such as body fluids or natural waters are very complex; in some cases, one analyte might enhance the luminol reaction while another inhibits it and the resulting signal is a combination of effects that is difficult to interpret. The situation is rather less difficult in process analytical chemistry, where there may be one and only one expected analyte. Coupling the chemiluminescence reaction post-column with a separation step (liquid chromatography or capillary electrophoresis) (ADD LINKS) can overcome interferences and give fmol-pmol detectivity. Labelling of sample components with luminol before separation can achieve the same end. Selectivity can also be provided by coupling the luminol reaction with enzymatic reactions or with antibody detection or with recognition by molecularly imprinted polymers.[3]
Many analogues of luminol have been synthesized;[1] some of them give more intense chemiluminescence than luminol itself but only if the modifications are restricted to the benzenoid ring of the luminol molecule. Changes to the heterocyclic ring abolish chemiluminescence. Phthalic hydrazide (luminol without the amine substituent) is not chemiluminescent except in aprotic solvents.
B1b. Mechanism
[edit | edit source]
Figure B1.1 – One- and two-electron routes of primary oxidation of luminol leading to secondary oxidation and chemiluminescence.
A mechanism for the oxidative chemiluminescence of luminol has been proposed by Roswell and White;[1] some of the individual steps have been studied by Lind, Merenyi and Eriksen.[4] Figure B1.1 is a flow chart of the mechanism; the structures of the main chemical species involved in the oxidation of luminol and the abbreviations for them used in the text are shown. The model proposes two step formation of luminol diazaquinone hydroperoxide anions (LOOH–), which spontaneously decompose (via a tricyclic endoperoxide transition state) to form dinitrogen and excited 3-aminophthalate anions that luminesce. The quantum yield of luminol oxidation by this route is high giving good analytical sensitivity.
b(i) Primary oxidation of luminol
[edit | edit source]The hydroperoxide intermediate (LOOH–) is formed in aqueous solution by the primary oxidation of the luminol monoanion (LH–) to a radical (L•–) followed by the addition of superoxide (O2•–) or by primary oxidation to diazaquinone (L) followed by addition of hydrogen peroxide anions (HO2–).[5]
(a) Luminol (LH2) exists in aqueous solutions at pH 10.0 as monoanions (LH–), which undergo one-electron oxidation, e.g., by hydroxyl radicals (HO•, E0 = +2.8 V), to form rapidly (k = 9.7 x 10−9 dm3 mol−1 s−1) diazasemiquinone radicals (L•–):
1) LH– – e– → LH• (e.g., LH– + HO• → L•– + H2O)
(b) Two-electron oxidation of luminol monoanion, e.g., with hydrogen peroxide, gives diazaquinone (L),
2) LH– – 2e― → L + H+ (e.g., LH– + H2O2 → L + H2O + HO–)
Two-electron oxidation occurs at the start of the luminol-hydrogen peroxide reaction. There is no superoxide present until hydrogen peroxide, competing with luminol for the hydroxyl radical, is converted to HO2•, which rapidly deprotonates to O2•– at high pH (pKa = 4.8):
3) H2O2 + HO• → O2•– + H3O+ Hydroxyl radicals reacting with luminol convert the monoanions (LH–) to L•– or LH•, depending on the pH; this is a one-electron oxidation process. Transfer of a second electron to form diazaquinone occurs only in the absence of superoxide, which otherwise would react with L•– or LH• to form luminol diazaquinone hydroperoxide anions (LOOH–).
The primary oxidation step usually determines the rate of light emission, so luminol chemiluminescence effectively measures the power of the oxidant to bring about this reaction but other factors also affect the rate of primary oxidation. Light emission from the reaction between luminol and hydrogen peroxide can be induced by the presence of cobalt(II) at concentrations low enough to be regarded as catalytic and it has been proposed that cobalt(II)-peroxide complex ions bring about the primary oxidation of luminol.[6]
b(ii) Secondary oxidation of luminol
[edit | edit source]In analytical luminol chemiluminescence, the initial oxidation of luminol is the rate-determining step. But chemiluminescence also depends on the availability of superoxide or hydroperoxide ions for secondary oxidation. So experiments have been performed using pulse radiolysis to bring about primary oxidation, allowing the rate of secondary oxidation to be studied in the pauses between the pulses. Protonated diazasemiquinone radicals (LH•) formed by one-electron primary oxidation add to superoxide radicals (O2•–) to form the diazaquinone hydroperoxide (LOOH–):
1) LH•– + O2•– → LOOH–
This reaction consumes superoxide radical anions and, in the presence of a large excess of hydrogen peroxide, the major part of LH•. LH•, however, can also recombine with itself. In the absence of superoxide, all luminol radicals are consumed by recombination, at least 80% of which is accounted for by dimerization. Diazaquinone (L), the product of two-electron primary oxidation of luminol, is converted to the peroxide by the addition of hydroperoxide anions:
2) L + HO2– → LOOH–
b(iii). Decomposition of hydroperoxide intermediate
[edit | edit source]Secondary oxidation is followed by the decomposition of the cyclic hydroperoxide intermediate to 3-aminophthalate, which emits light on relaxation to the ground state.
LOOH– → 3-aminophthalate + N2 + hν
The basic peroxide adduct (LOOH–) decomposes to form the excited state of the aminophthalate emitter, while the protonated adduct undergoes a non-chemiluminescent side reaction which forms a distinct yellow product, the so-called “dark reaction”.[7] The absorbance spectrum of LOOH– decays at the same rate as does the light emission. Emission intensity increases with pH up to a maximum at about pH = 11, reflecting increasing dissociation of H2O2 into its anion and the diminishing importance of the dark reaction . Decreased light output above pH 11 reflects diminished fluorescent quantum yield (ΦFL) of the emitter.
b(iv) Determination of chemiluminescence quantum yield
[edit | edit source]Lind and Merényi[8] have measured the light yield of several chemiluminescent reactions of luminol undergoing one-electron oxidation by hydroxyl radicals of radiolytic origin. Using the transfer of 100 eV from ionising particles to aqueous media, it becomes possible to calculate ΦCL by ΦCL = G(hν)/ ΦCLGOH. They propose as a standard for luminol chemiluminescence initiated by pulse radiolysis a system consisting of 10−3 mol dm−3 aqueous luminol at pH = 10.0 saturated with 10% O2 and 90% N2O. Having defined a standard with a well-defined light output, it then becomes possible to determine the chemiluminescence quantum yield of any other luminol reaction relative to the standard. This has been done by Merényi and Lind[9] by plotting integrated light intensity as a function of radiolytic dose (which has a linear relationship with hydroxyl radical concentration). The light yields and hence the relative quantum yields are obtained by comparing the slopes of the plots.
B1c. Oxidants used in luminol chemiluminescence
[edit | edit source]c(i). Hydrogen peroxide
[edit | edit source]Hydrogen peroxide is analytically the most useful oxidant of luminol, but requires the catalytic effect of an electrode, a metal ion or an enzyme. For example, it reacts readily with luminol in an aqueous medium in the presence of a cobalt(II) catalyst. This reaction is a very effective bench demonstration of chemiluminescence, using equal volumes of 0.1 mol dm−3 hydrogen peroxide and 1.0 x 10−3 mol dm−3 luminol in 0.1 mol dm−3 carbonate buffer (pH between 10 and 11). Some metal ions, used to catalyse the oxidation of luminol e.g. iron(II), react with hydrogen peroxide: Fe2+ + H2O2 → Fe3+ + HO• + HO– to generate hydroxyl radicals (Fenton Reaction), which have very powerful oxidizing properties and can therefore bring about the primary oxidation of luminol. But they also react with hydrogen peroxide (equation1) and hydroperoxide ions (equation 2): 1) H2O2 + HO• → O2•– + H3O+ 2) HO2– + HO• → O2•– + H2O The consumption of hydroxyl radicals in these reactions diminishes the rate of primary oxidation, but the generation of superoxide increases the rate of secondary oxidation.
c(ii). Oxygen
[edit | edit source]The standard reduction potential (E0) of luminol radicals to monoanions (LH• + e− → LH−) has been determined to be +0.87 V.[10] Oxidation by molecular dioxygen (E0 = 1.229 V) is thermodynamically possible but in aqueous solutions, the reactivity is undetectably low at any pH (k = 10−8 dm3 mol−1 s−1) and so the reaction is not useful for primary oxidation. It is widely believed that air-saturated luminol solutions are indefinitely stable in the dark, even at pH = 14. In spite of this, the oxidation of luminol by dissolved oxygen in aqueous solutions is frequently reported. It is likely that what is referred to in these cases is oxidation by oxygen radicals, which may be formed from molecular dioxygen by suitable reductants such as metal ions. This phenomenon is discussed in chapter D10 along with other cases in which oxygen radicals act as chemiluminescence reagents (LINK).
In dimethylsulfoxide (DMSO) solutions, luminol exists as dianions and reacts with dissolved oxygen in the presence of a strong base with intense chemiluminescence.[1] The rate constant for the reaction is about 50 dm3mol−1s−1;[10] the rate constant for the corresponding reaction between oxygen and luminol dianions in aqueous alkali is 10−2 dm3 mol−1 s−1. Because the conditions of the reaction in DMSO solution are relatively simple, the phenomenon has found great favour as a demonstration,[11] for a spatula measure of luminol in a bottle of alkaline DMSO will react on shaking at room temperature. However, while the oxidation of luminol in aqueous solution is very widely used analytically, there are no established analytical procedures making use of the reaction in dimethylsulfoxide, dimethylformamide or other organic solvents but the effect of a range of metal complexes on the reaction in DMSO has been investigated[12][13] The emitter (3-aminophthalate ion) tautomerizes in aprotic solvents such as DMSO to a quinonoid form that gives maximum emission at 510 nm; this tautomer is favoured by the pairing of luminol anions with metal cations (e.g., Na+ or K+). If luminol is oxidized in mixed solvents, there is less emission at 425 nm (reduced ion pairing) and more at 510 nm than in aqueous media. Also in mixed solvents there is less 425 nm emission in chemiluminescence than in fluorescence because in chemiluminescence the fraction of ion-pairs is determined by the transition-state rather than by the ground-state equilibrium as in fluorescence.[1]
c(iii). Higher oxidation states of manganese
[edit | edit source]Permanganate ions are thermodynamically easily capable (E0 = 1.70 V) of oxidizing luminol. A flow injection analysis of paracetamol in pharmaceutical preparations based on inhibition of luminol-permanganate chemiluminescence has been reported.[14] A little earlier, an imaginative biosensor for urea had been fabricated, in which ammonium carbonate generated by urease-catalysed hydrolysis was used to release luminol from an anion-exchange column to react with permanganate eluted from a second column, producing chemiluminescence.[15] A steady stream of novel applications of the luminol-permanganate system followed.
Oxidation of luminol by alkaline potassium permanganate produces manganate(VI) ions, which further react with luminol causing chemiluminescence. This phenomenon, termed by the authors ‘’’second chemiluminescence (SCL)’’’ has been applied in an assay for nickel(II) ions.[16] In a suitable flow injection manifold, dilute solutions of alkaline luminol and of aqueous potassium permanganate are mixed and allowed to react for a long enough time for the resulting chemiluminescence to drop to a stable minimum close to zero. The sample is then injected into the mixture and, if nickel ions are present, light emission recommences and rapidly rises to a well-defined peak before returning to baseline intensity. Optimum intensity of the second chemiluminescence was obtained by using a tenfold excess of luminol (over potassium permanganate) in 0.1 mol dm−3 aqueous sodium hydroxide and injecting sample at pH 5.10; linear relationship with nickel(II) concentration was established and the detection limit was 0.33 μg dm−3. Numerous divalent and trivalent metal ions and nitrate ions were found to interfere with the determination. The mechanism of the luminol-manganate(VI) chemiluminescence appears to be the same as that for other luminol oxidations, with the production of excited 3-aminophthalate ion emitting at 425 nm. But oxidations both by permanganate and manganate(VI) can lead to the formation of excited manganese(II), which would be an additional source of chemiluminescence. Unfortunately, in the work described, the chemiluminescence spectrum was observed only up to 490 nm, overlooking such possible contributions to the signal.
c(iv) Silver(III)
[edit | edit source]A fairly stable silver(III) complex anion, diperiodatoargentate(III) (DPA), [Ag (H2IO6)(OH)2]2−, can be readily synthesized.[17] A new chemiluminescence reaction between luminol and diperiodatoargentate has been observed in alkaline aqueous solution.[18][19] The emission of light from this reaction is strongly enhanced by iron nanoparticles and the intensity is further increased by the addition of aminophylline.[20] This forms the basis for an assay in which the chemiluminescence signal has a linear relationship with aminophylline concentration in human serum over the range 1.0 x 10−8 to 2.0 x 10−6 mol dm−3. The detection limit is 9.8 x 10−9 mol dm−3. The relative standard deviation at 8.0 x 10−7 mol dm−3 is 4.8% (n = 10).
Penicillin antibiotics have also been found to enhance luminol-silver(III) complex chemiluminescence, which has formed the basis for a sensitive flow injection assay for these drugs in dosage forms and in urine samples. In optimized conditions, the detection limit for benzylpenicillin sodium is reported to be 70 ng cm−3, for amoxicillin 67 ng cm−3, for ampicillin 169 ng cm−3 and for cloxacillin sodium 64 ng cm−3.[21]
The maximum wavelength of the light emitted is about 425 nm,[18] which is the usual chemiluminescence from excited 3-aminophthalate, produced by luminol oxidation. This implies that the silver(III) complex is capable of bringing about both the primary and secondary oxidation of luminol as proposed by Shi ‘’et al.’’ who postulate one-electron primary oxidation of two luminol molecules by each diperiodatoargentate. Perhaps two-electron oxidation of one luminol molecule is more likely. The reduction potential of diperiodatoargentate(III) ion is +1.74 V,[22] high enough for two-electron oxidation to convert water into hydrogen peroxide (ε0 = -1.763 V; Nernst equation indicates a millimolar H2O2 equilibrium concentration). This provides a possible mechanism for secondary as well as primary oxidation.
B1d. Electrochemiluminescence
[edit | edit source]The instrumentation of electrochemiluminescence (ECL) is dealt with in chapter D7. The resulting reaction pathways lend themselves to control of emission by varying the applied voltage or the electrode selected and are applicable to near- neutral (pH 8.0 to 8.5) aqueous solutions such as biological fluids, whereas luminol chemiluminescence usually occurs in strongly alkaline or non-aqueous solutions. It has been proposed that luminol is first oxidized at the electrode surface and, on subsequent reaction with hydrogen peroxide, the chemiluminescence quantum yield (see chapter A1 ADD LINK) is enhanced.[23][24] Typical of the early applications is an assay of lipid hydroperoxides using ECL at a vitreous carbon electrode.[25] With applied voltages of 0.5-1.0 V, luminol monoanion loses one electron giving diazasemiquinone, which dismutes to produce diazaquinone, which reacts quantitatively with the analyte. At applied voltages above 1.0 V, the –NH2 of diazaquinone and the analyte itself are oxidized giving respectively –NH• and superoxide, which causes an interfering signal. The detection limit in optimized conditions was 0.3 nmol at S/N = 1.5. Using a voltage of 0.5-1.0 V applied to a platinum electrode, both methyl linoleate hydroperoxide (MLHP) and luminol are oxidized; the detection limit for MLHP is 0.1 nmol at S/N = 2.5. There was no emission from the closely related methyl hydroxyoctadecadienoate (a reduction product of linoleic acid hydroperoxide). The inhibition of ECL signals from luminol oxidation can be used as a method of determination of inhibitors. A recent example is the determination of melamine in dairy products and in tableware.[26] Using low voltage scan rates in phosphate buffer at high pH, ECL is observed at 1.47 V and there is a linear (r2=0.9911) decrease of ECL proportional to the logarithm of the melamine concentration over the range 1 to 100 ng cm−3. The limit of detection is 0.1 ng cm−3 with high recovery. The signal arises from the reaction with luminol of reactive oxygen species (from the electrooxidation of hydroxyl ions) that are eliminated by melamine. Modification of electrodes is now a well-established way of controlling ECL and in recent years the use of nanomaterials for this purpose has grown in importance. An example involving luminol is the modification of a gold electrode by applying a composite of multi-wall carbon nanotubes and the perfluorosulfonate polymer Nafion.[27] In the course of cyclic voltammetry in carbonate buffer, three ECL peaks were obtained, up to 20 times as intense as with the unmodified electrode; in each case the emitter was identified as 3-aminophthalate anion, indicating that the improvement was due to electrode efficiency rather than to any change in the chemistry of the system.
ECL immunosensors have been fabricated that have been successfully applied to the determination of human immunoglobulin G (hIgG) in serum. The primary antibody, biotin-conjugated goat anti-hIgG, is first immobilized on an electrode modified with streptavidin-coated gold nanoparticles (AuNPs). The sensors are sandwich-type immunocomplexes formed by the conjugation of hIgG to a second antibody labelled with luminol-functionalized AuNPs. ECL is generated by a double potential step in carbonate buffer containing 1.0 mmol dm−3 hydrogen peroxide. Many luminol molecules are attached to the surface of each AuNP and act as multiple sources of light emission from each antibody molecule. The amplification of ECL in this way, linked to the analyte by the biotin-streptavidin system, leads to greatly enhanced signals. The limit of detection is 1 pg cm−3 (at S/N = 3), which surpasses the performance of all previous hIgG assays.[28]
The surface of a glassy carbon electrode was modified by producing on it L-cysteine reduced graphene oxide composites onto which AuNPs were self-assembled. Cholesterol oxidase (ChOx) was subsequently adsorbed on the AuNP surface to form a cholesterol biosensor with satisfactory reproducibility, stability and selectivity. The AuNPs increase the surface area of the electrode, hence permitting a higher ChOx loading, and provide a nanostructure more favourable to ECL, improving analytical performance. The linear response to cholesterol of the sensor extends over the range 3.3 x 10−6 to 1.0 x 10−3 mol dm−3 and the limit of detection is 1.1 x 10−6 (at S/N = 3).[29]
A poly(luminol-3,3',5,5'-tetramethylbenzidine) copolymer manufactured by electropolymerization on screen-printed gold electrodes greatly improves the ECL of hydrogen peroxide. A cholesterol biosensor suitable for the analysis of serum samples was fabricated by immobilization of cholesterol oxidase onto the polymer. Under optimized conditions, the biosensor has a linear dynamic range of 2.4 x 10−5 to 1.0 x 10−3 mol dm−3 with a limit of detection of 7.3 x 10−6 mol dm−3. Precision (measured as relative standard deviation) was 10.3% at 5.0 x 10−4 mol dm−3 and the method has the additional advantages of low cost and high speed.[30]
References
[edit | edit source]- ↑ a b c d e Roswell D F and White E H, The chemiluminescence of luminol and related hydrazides, In: Fleischer S and Fleischer B (eds.), Meth. Enzymol. , 1978, 57, 409.
- ↑ Sakura S, Anal Chim Acta, 1992, 262(1), 49.
- ↑ a b Marquette CA and Blum LJ, Applications of the luminol chemiluminescent reaction in analytical chemistry, Anal Bioanal Chem, 2006, 385, 546-554.
- ↑ Lind J, Merényi G and Eriksen T E,Chemiluminescence Mechanism of Cyclic Hydrazides Such ass Luminol in Aqueous Solutions, J. Am. Chem. Soc., 1983, 105, 7655.
- ↑ Merényi G, Lind J and Eriksen T E, Luminol Chemiluminescence: chemistry, Excitation, Emitter, J. Biolumin. Chemilumin., 1990, 5, 53.
- ↑ Burdo T G and Seitz W R, Mechanism of Cobalt Catalysis of Luminol Chemiluminescence, Anal. Chem., 1975, 47(9), 1639-1643.
- ↑ Merényi G and Lind J S, Role of a peroxide intermediate in the chemiluminescence of luminol. A mechanistic study, J. Am. Chem. Soc., 1980, 102, 5830.
- ↑ Lind J S and Merényi G, Determination of the chemiluminescence quantum yield of luminol in rapid chemical reactions, Chem. Phys. Lett., 1981, 82(2), 331-334.
- ↑ Invalid
<ref>tag; no text was provided for refs namedMerényi-Lind1980 - ↑ a b Merényi G, Lind J, Shen X and Eriksen T E, Oxidation Potential of Luminol: Is the Autoxidation of Singlet Organic Molecules an Outer-Sphere Electron Transfer?, J. Phys. Chem., 1990, 94, 748-752.
- ↑ Schneider H W, A New, Long-Lasting Luminol Chemiluminescent Cold Light, J. Chem. Ed., 1970, 47, 519-522.
- ↑ Golcu A, Tumer M, Demirelli H and Wheatley R A, Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: synthesis, characterization, properties and biological activity, Inorg. Chim. Acta , 2005, 358, 1785-1797.
- ↑ Golcu A, Wheatley R A, Demirelli H, Tumer M and Dolaz M, Investigations into the Inhibition of Luminol Chemiluminescence by some Novel Metal Complexes, Curr. Anal. Chem., 2010, 6(2), 144-153.
- ↑ Easwaramoorthy, D, Yu, YC and Huang, HJ, Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction, Anal. Chim. Acta, 2001, 439 (1), 95-100.
- ↑ Qin W, Zhang ZJ and Peng YY, Plant tissue-based chemiluminescence flow biosensor for urea, Anal. Chim. Acta, 2000, 407 (1-2), 81-86.
- ↑ Li L N, Li N B and Luo H Q, a new chemiluminescence method for the determination of nickel ion, Spectrochimica Acta Part A, 2006, 64, 391-396.
- ↑ Shen S, Shi H and Sun H, Kinetics and mechanism of oxidation of the drug mephenesin by bis(hydrogenperiodato)argentate(III) complex anion, International Journal of Chemical Kinetics , 2007, 39(8), 440-446.
- ↑ a b Shi H, Xu X, Ding Y , Liu S, Li L, Kang W, Determination of cortisol in human blood sera by a new Ag(III) complex–luminol chemiluminescent system, Anal. Biochem., 2009, 387, 178–183.
- ↑ Yang C, Zhang Z and Wang J, New luminol chemiluminescence reaction using diperiodatoargentate as oxidate for the determination of amikacin sulfate, Luminescence, 2010, 25(1), 36-42. DOI: 10.1002/bio.1140
- ↑ Rezei B, Ensafi A A, Zarei L, Fast and sensitive chemiluminescence assay of aminophylline in human serum using luminol-diperiodatoargentate(III) system catalyzed by coated iron nanoparticles, Spectrochim. Acta A , 2012, 90, 223-229.
- ↑ Ma L, Kang W J, Xu X D, Niu L M, Shi H M and Li S, Flow-injection chemiluminescence determination of penicillin antibiotics in drugs and human urine using luminol-Ag(III) complex system, J. Anal. Chem., 2012, 67(3), 219-225.
- ↑ Savanur A P, Lamani S D, Nandibewoor S T and Chimatadar S A, Mechanistic Investigations of Uncatalysed and Osmium(VIII) Catalysed Oxidation of Chlorpheniramine an Antihistamine Drug by Diperiodatoargentate(III) Complex in Aqueous Alkaline Medium: A Comparative Kinetic Approach, J. Chem. Pharm. Res., 2011, 3(6), 1061-1088.
- ↑ Marquette CA and Blum LJ, Anal. Chim. Acta , 1999, 381(1), 546-554.
- ↑ Sakura S, Determination of lipid hydroperoxides by electrochemiluminescence, Anal. Chim. Acta, 1992, 262(1), 49.
- ↑ Sakura S and Terao J, Determination of lipid hydroperoxides by electrochemiluminescence, Anal. Chim. Acta, 1992, 262 (1), 59-65.
- ↑ Jing W, Lu S Y, Li X J, Jiang X F, Chen M S, Liang M, Tang X, Xu C M and Chen J Q, Determination of Melamine in Dairy Products and Melamine Tableware by Inhibition Electrochemiluminescent Method, Chin. J. Chem., 2011, 29(8), 1601-1605.
- ↑ Dong Y P, Electrogenerated chemiluminescence of luminol at a carbon nanotube-perfluorosulfonate polymer (Nafion) modified gold electrode, J. Lumin., 2010, 130(8), 1539-1545.
- ↑ Tian D Y, Duan C F, Wang W and Cui H, Ultrasensitive electrochemiluminescence immunosensor based on luminol functionalized gold nanoparticle labelling, Biosensors and Bioelectronics , 2010, 25(10), 2290-2295.
- ↑ Zhang M H, Yuan R, Chai Y Q, Chen S H, Zhong H A, Wang C and Cheng Y F, A biosensor for cholesterol based on gold nanoparticles-catalyzed luminol electrogenerated chemiluminescence, Biosensors and Bioelectronics , 2012, 32(1), 288-292.
- ↑ Ballesta-Claver J, Velazquez P S, Valencia-Miron M C and Capitan-Vallvey L F, SPE biosensor for cholesterol in serum samples based on electrochemiluminescent luminol copolymer, Talanta, 2011, 86, 178-185.
Lophine and pyrogallol
B2. Lophine and pyrogallol
[edit | edit source]These are the earliest-known chemiluminescence reagents. Lophine (2,4,5-triphenyl-1H-imidazole) exhibits lemon yellow chemiluminescence in solution and is one of the few long-lasting chemiluminescent molecules. It forms dimers that have piezochromic and photochromic properties. It has been proposed as an analytical reagent for trace metal ion detection.[1] Lophine chemiluminescence was discovered by B. Radziszewski in 1877.
Pyrogallol is determined by means of chemiluminescence at 500 nm which accompanies the oxidation by hydrogen peroxide of autoxidized pyrogallol in the presence of chromium(III) and formaldehyde. Using air-segmented continuous flow analysis, the LOD (3s) was 6.0 × 10−9 mol dm−3 and the calibration was linear up to 10−4 mol dm−3. The method has the potential to be extended to other phenols[2] The chemiluminescent oxidation of pyrogallol has been known for more than one hundred years.
References
[edit | edit source]- ↑ Macdonald A, Chain K W and Nieman T A, Lophine chemiluminescence for metal ion detection, Anal. Chem., 1979, 51(13), 2077-82
- ↑ Kearney N J, Bridgeland E S, Jewsbury R A, Martin N D, Kelly S J and Korn S R, Determination of Pyrogallol Using Continuous Flow With Chemiluminescence Detection, Anal. Comm., 1996, 33, 241-243
Luciferins
B3. Luciferins
[edit | edit source]Luciferases are enzymes that catalyse light-emitting reactions in living organisms - bioluminescence. They occur in several species of firefly and in many species of bacterium. Firefly Luciferases are extracted by differential centrifugation and purified by gel filtration. Lyophilised luciferase with added stabiliser keeps for several months at ―4 °C.
Luciferins are substrates of luciferases . Firefly luciferin emits at 562 nm on reaction with oxygen, catalysed by luciferase in the presence of adenosine triphosphate (ATP) and magnesium ions, emission being directly proportional to luciferin concentration over the range 0.01-1000 nmol dm―3. The ATP dependence of firefly luciferin bioluminescence is exploited in many ATP determinations and assays for the products of enzymatic reactions that utilize or produce ATP, e.g., kinases and substances involved in reactions catalysed by them.
The crustacean Cypridina hilgendorfii has a luciferin of very different chemical structure, but the mechanism of its bioluminescence is the same as that of the firefly except that no co-factor is required. Analogues of Cypridina luciferin have also been synthesised and used to detect superoxide of pathological origin. Scavengers of superoxide radicals, e.g., tea leaf catechins, quench Cypridina chemiluminescence, enabling their antioxidant activities to be conveniently measured.

Figure B3.1 – Principle of bacterial bioluminescence, in which light is emitted by the oxidation of a long-chain fatty aldehyde by flavine mononucleotide, which is regenerated in a coupled reaction. NAD(P)H, nicotinamide adenine dinucleotide (phosphate; FMN, flavine mononucleotide.
Luminous bacteria are found widely in marine environments. Bacterial luciferase, which acts in accordance with the outline mechanism shown in Figure B3.1, does not have a luciferin substrate as such. Instead the light emission comes from a complex of luciferase, flavine mononucleotide and a long-chain fatty aldehyde.[1] Thus bacterial bioluminescence is associated with a pyridine nucleotide rather than with the adenine nucleotide involved in firefly bioluminescence.
References
[edit | edit source]- ↑ McCapra F in Turner A P F, Karube I and Wilson G S, Biosensors: Fundamentals and Applications, Oxford, Oxford University Press, 1987. p 617.
Lucigenin and coelenterazine
B4. Lucigenin and coelenterazine
[edit | edit source]Lucigenin and related 9,9/-diacridinium salts give an intense blue-green emission when oxidized by alkaline hydrogen peroxide. The major chemiluminescence emitter is postulated[1] to be N-methyl acridone (blue light), produced via a peroxide, with other excited molecules involved. The reaction is catalysed by pyridine, piperidine, ammonia or osmium tetroxide. A proposed mechanism explains the chemiluminescence of oxidized acridinium salts by the formation of excited peroxide intermediates.
Lucigenin is used in a wide variety of assays, especially those involving enzymatic production of hydrogen peroxide, and as a label in immunoassays. It reacts with various reductants, including those present in normal human blood,[2] such as glutathione, uric acid, glucuronic acid, creatinine, ascorbic acid and creatine. The chemiluminescence intensity for a mixture of these analytes is equal to the sum of the intensities, measured separately for each analyte present. Metal ions – iron(III), manganese(II) and copper(II) – also contribute to the chemiluminescence and so must be regarded as interferents. Lucigenin is also affected by a very wide range of other metal ions,[3] both enhancers and inhibitors. The most effective enhancers are osmium (VIII), cobalt(II), ruthenium(III), iron(II) and iron(III) and the most effective inhibitors are europium(III), thorium(IV), ytterbium(III), terbium(III) and manganese(II). Among the enhancers, effective enhancement seems to be associated with low detection limit but this association is much less pronounced among the inhibitors.
Lucigenin chemiluminescence has been important for the determination of superoxide.[4] The mechanism for the lucigenin-superoxide reaction is believed to be:
(B4.1) Reduction to cation radical: Luc2+ + e- → Luc•+
(B4.2) Coupling to yield dioxetane: Luc•+ + O2•– → LucO2
(B4.3) Decomposition of dioxetane to N-methylacridone: LucO2 → NMA* + NMA
(B4.4) Chemiluminescence: NMA* → NMA + light
The credibility of lucigenin detection of superoxide has been questioned because of the evidence (disputed) for a process called redox cycling in which lucigenin reacts with oxygen to form more superoxide, leading to the amount of superoxide being overestimated. As a result, coelenterazine (a luminophore from the coelenterate Aequorea), became a more favoured probe for superoxide; although this also offered improved selectivity for superoxide, it was not entirely specific. Attention has therefore shifted to assays using Cypridina luciferin analogues (see chapter B3) to detect superoxide.
References
[edit | edit source]
Dioxetanes and oxalates
B5. Dioxetanes and oxalates
[edit | edit source]Peroxy-oxalate chemiluminescence (PO-CL) was first reported in 1963 as a very weak bluish-white emission from oxalyl chloride, Cl-CO.CO-Cl, on oxidation by hydrogen peroxide; a similar blue emission occurs from related oxalyl peroxides. Much more intense emission is obtained in the reaction between aryl oxalates and hydrogen peroxide in the presence of a fluorophore; it is this version of the reaction that is analytically useful.[1][2] Liquid chromatography is a major area of application.[3]
Because in PO-CL analysis, the analyte is an added fluorophore to which energy is transferred, the various applications have much in common. The rate of PO-CL depends especially on pH and on the presence of a nucleophilic base catalyst for ester hydrolysis. Aryl oxalates differ in the effect of pH on the intensity and decay of the chemiluminescence. They also differ in their solubilities, which affects their usefulness as detection reagents for HPLC. There are wide variations in their stabilities in the presence of hydrogen peroxide, so some are more suitable than others for premixing with the oxidant. Taking all these things into account, Honda et al. proposed that the preferred oxalate varied with pH as follows:
- <2: bis(pentafluorophenyl)
- 2 to 4: bis(2-nitrophenyl)
- 4 to 6: bis(2,4-dinitrophenyl)
- 6 to 8: bis(2,4,6-trichlorophenyl)
- >8: bis(2,4,5-trichloro-6-pentyloxycarbonylphenyl)
PO-CL is thought to follow a chemically initiated electron exchange luminescence (CIEEL) mechanism as proposed by Koo and Schuster.[4] An electron is transferred from the fluorophore to an intermediate, which, as it decomposes, transfers it back again; as a result the fluorophore is raised to an excited state and subsequently radiates. In support of this it has been demonstrated that the relative excitation yields of different fluorescers have a significant negative correlation with their oxidation potentials – in other words, the more difficult it is to oxidize the fluorescer, the lower its probability of excitation. High chemiluminescence intensity can be predicted if a fluorescer has a low singlet excitation energy ; a low oxidation potential is at least as important. The formation of a linear peroxide intermediate, ArO-CO.CO-OOH, which decomposes to radical ion-pairs comprising the fluorophore and a carbon dioxide molecule, has also been proposed as the mechanism of energy transfer. Background emission in the absence of a fluorophore occurs at 450 nm (which could be carbon dioxide) and at about 550 nm (which varies with the aryl group and could be due to an excited carbonyl intermediate containing the aryl group). Dioxetanes luminesce on warming, producing excited carbonyl compounds and the may have a role in PO-CL. However, decomposition of 1,2-dioxetanedione into carbon dioxide, though possible, is unlikely to be the sole source of the emission as the chemiluminescence depends on the electronegativity of the aryl group, so is unlikely to arise from an intermediate that would be the same whatever the aryl group.
References
[edit | edit source]- ↑ Townshend A, Solution Chemiluminescence - Some Recent Analytical Developments, Analyst, 1990, 115, 495-500.
- ↑ Robards K and Worsfold P J, Analytical applications of liquid-phase chemiluminescence, Anal. Chim. Acta, 1992, 266 (1992), 147-173.
- ↑ Kwakman P J M and Brinkman U A Th, Peroxyoxalate chemiluminescence detection in liquid chromaography, Anal. Chim. Acta, 1992, 266, 175 - 192.
- ↑ Koo J-Y and Schuster G B, Chemically initiated electron exchange luminescence. A new chemiluminescence reaction path for organic peroxides, J. Am. Chem. Soc., 1977, 99, 6107.
Organic peroxides and lipid peroxidation
B6. Organic peroxides and lipid peroxidation
[edit | edit source]Metal ions such as iron decompose organic peroxides and hydroperoxides into free radicals;[1] the rate of formation varies very much with different metal complexes and peroxides. The chemiluminescence intensity is directly proportional to the concentration of hydroperoxide. Cyclic organic peroxides include dioxetanes which have been disussed in connection with the peroxy-oxalate reaction. The mechanisms involved in the decomposition of 1,2-dioxetanes and analogous peroxides are: (i) unimolecular decomposition into excited state carbonyl compounds; (ii) intramolecular or intermolecular CIEEL (Chemically Initiated Electron Exchange Luminescence).
Lipid peroxidation is a process of great interest, especially in biochemical research, as it is associated with damage to biological cell membranes and has a putative role in pathological phenomena such as aging, cancer and other degenerative conditions. The process is a radical chain reaction that produces an ultraweak chemiluminescence signal. It has been proposed that in cells, the major excited species responsible for light emission are triplet carbonyls and singlet oxygen, which arise through the decomposition of hydroperoxides. Initiators such as hydroxyl radicals (•OH) remove hydrogen from unsaturated fatty acids (LH) to produce lipid radicals (L•):
LH + •OH → L• + H2O
which react with atmospheric oxygen to form lipid peroxyl radicals (LO2•):
L• + O2 → LO2•
that recombine to generate the excited products (P):
LO2• + LO2• → P* → P + Фhν
(h = Planck’s constant and ν = frequency of emitted light).
The emission intensity is determined by the quantum yield (Ф), which is low for lipid peroxidation, depending on the rate of processes competing with light emission for the deactivation of the lowest excited singlet state. Because the associated chemiluminescence is weak, it is useful to enhance the emission intensity using fluorescent dyes, as discussed in chapter 16.
References
[edit | edit source]- ↑ Noguchi N and Niki E, Free Radical Research, 1995, 23(4), 329
Manganese
B7. Manganese
[edit | edit source]Manganese (VII) in the form of potassium permanganate has been used as a chemiluminescence reagent for several decades. A broad band of red light is emitted on reaction with over 270 compounds in acidic solution.[1] Among the organic analytes are morphine and a wide range of other pharmaceuticals, phenolic substances, amines and hydrazines in addition to well-known reductants such as ascorbic acid and uric acid. Proteins and amino-acids are also known to reduce permanganate with chemiluminescence. Inorganic analytes include sulfur dioxide and sulfites, hydrogen sulfide, hydrogen peroxide, hydrazine and iron(II). Chemiluminescence intensity is a linear function over a very wide range of concentration, but varies considerably for different analytes. It is also affected by anions present so that acidification with sulfuric acid gives a better signal than hydrochloric, nitric or perchloric acids. Considerable signal enhancement occurs in the presence of polyphosphates; these are unstable at low pH but hexametaphosphate is more stable than the others. In a number of cases, chemiluminescence is enhanced by the presence of an ancillary reductant such as formic acid or, especially, formaldehyde. Manganese(II) is sometimes a useful signal enhancer. Fluorophores such as quinine, riboflavin or rhodamine B have also been used but sometimes give a high blank signal and a reduced signal to noise ratio.
The emitting species is an electronically excited manganese(II) species, as has been confirmed by a direct comparison of the laser-induced photoluminescence of manganese(II) chloride with the chemiluminescence from reaction of sodium borohydride with acidic potassium permanganate.[2] In many cases where permanganate is used in the presence of fluorescent compounds, e.g. enhancers or reaction products, energy transfer to the efficient fluorophore has been proposed on the basis of spectral distributions that match those obtained using other oxidants; in most cases, however, the red emission characteristic of manganese(II) is also produced and can make a significant contribution to the total light output,[3] especially in the presence of polyphosphate.
More recently, manganese(III) and manganese(IV) have been explored as chemiluminescence reagents.[4] As with the +VII oxidation state, these produce on reaction with a wide range of molecules an excited manganese(II) species that emits light, but differ markedly in terms of selectivity. They also possess characteristics that provide new avenues for detection, such as the immobilisation of solid manganese dioxide, the production of colloidal manganese(IV) nanoparticles and the electrochemical generation of manganese(III).
A brown, transparent, stable solution of manganese(IV) can be prepared by dissolving freshly precipitated manganese dioxide in 3M orthophosphoric acid. Using this reagent at about 1 x 10-4 M, analytically useful chemiluminescence has been reported for a growing list of compounds, often with nanomolar detection limits. Light emission is enhanced by up to 2 orders of magnitude in the presence of 0.2 – 3.0 M formaldehyde. Numerous pharmaceuticals have been determined in commercial formulations by this reaction in flow-injection assays. Detection of drugs and biomolecules in more complex matrices such as urine or serum requires coupling with an initial separation step such as HPLC.
Manganese(III) can be obtained by oxidation of manganese(II) or reduction of manganese(IV); it readily disproportionates into the +II and +IV states but can be stabilised by acidification, by complexation with anions or by adding manganese(II). The reduction of manganese(III) produces excited manganese(II) leading to emission of light of the same spectral characteristics as that emitted in permanganate or manganese(IV) chemiluminescence. On-line electrochemical generation of manganese(III) from manganese(II) has been applied to the chemiluminescence determination of a wide range of analytes, especially pharmaceuticals, with satisfactory selectivity and typically sub-micromolar limits of detection.
References
[edit | edit source]- ↑ Hindson B J and Barnett N W, Analytical applications of acidic potassium permanganate as a chemiluminescence reagent, Anal. Chim. Acta, 2001, 445, 1-19.
- ↑ Adcock JL, Francis PS, Smith TA and Barnett NW, The characteristic red chemiluminescence from reactions with acidic potassium permanganate: further spectroscopic evidence for a manganese(II) emitter, Analyst, 2008, 133(1), 49-51.
- ↑ Adcock JL, Francis PS and Barnett NW, Anal. Chim. Acta, 2009, 652(1-2), 303-307.
- ↑ Brown A J, Francis P S, Adcock J L, Lim K F and Barnett N W, Manganese(III) and manganese(IV) as chemiluminescence reagents:: A review, Anal. Chim. Acta, 2008, 624, 175-183.
Cerium
B8. Cerium
[edit | edit source]Cerium(IV)-based chemiluminescence systems involve the reduction of cerium(IV), which suggests that the emitter is a cerium(III) species. The chemiluminescence reaction is carried out in an acidic medium (generally sulfuric acid) and has been applied for the determination of substances of biological interest.[1] A few pharmaceuticals in dosage forms can reduce the cerium(IV) and produce luminescence directly. As a result, many flow-injection-chemiluminescence methods have been established for such species as naproxen, acetaminophen and fluphenazine hydrochloride. The sensitivity of the assays can be improved by increasing cerium (IV) concentration. Almost all cerium(IV) chemiluminescence systems need sensitization procedures to transfer the excited-state energy to a sensitizer, which then emits light of greater intensity. Thus most determinations involving cerium(IV) as the oxidant are indirect, based on the enhancement of chemiluminescence of the cerium(IV)-sulfite system by some analytes. This type of process is used to determine reducing compounds, such as cortisone, ofloxacin, nomoxacin, ciprofloxacin, lomefloxacin, flufenamic acid, mefenamic acid and salicylic acid.
Cerium(IV) chemiluminescence systems are very popular to determine sulfur-containing substances such as sodium-2-mercaptoethane, tiopronin, captopril, menadione sodium bisulfite and some sulfur-substituted benzamides but also other substances such as paraben, phenolic compounds (by LC), phentolamine, barbituric acid and erythromycin. In addition, light emission resulting from the chemical reaction of cerium(IV) with some mercapto-containing compounds in pharmaceutical preparations can be enhanced by certain fluorometric reagents such as quinine, rhodamine B and rhodamine 6G or by lanthanide ions such as terbium(III) and europium(III). Thus, a range of flow-injection chemiluminescence methods have been developed for determination of compounds of this kind.
References
[edit | edit source]- ↑ Chen J and Fang Y, Sensors, 2007, 7, 448-458.
Ruthenium
B9. Ruthenium
[edit | edit source]The chemiluminescence involving tris(2,2'-bipyridyl)ruthenium(II), [Ru(bpy)3]2+, is most interesting. It involves the oxidation of [Ru(bpy)3]2+ to [Ru(bpy)3]3+, which is followed by reduction with an analyte species to produce an emission of light, thus:
(B9.1) Oxidation: [Ru(bpy)3]2+ ― e― → [Ru(bpy)3]3+
(B9.2) Reduction by analyte: [Ru(bpy)3]3+ + e― → [Ru(bpy)3]2+*
(B9.3) Chemiluminescence: [Ru(bpy)3]2+* → [Ru(bpy)3]2+ + light (620 nm)

Figure B9.1 – The arrangement of the three 2,2'-bipyridine ligands about the central ruthenium atom in the complex ion tris(2,2'-bipyridyl)ruthenium(II); the nitrogen atoms occupy the corners of an octahedron.
Analytical usefulness depends on the emission of light of a measurable intensity that is clearly indicative of the analyte concentration. Chemiluminescence intensity depends on the efficiency and mechanism of the reduction step (eqn. B9.2). Common to all analytical applications of ruthenium chemiluminescence is the production of the oxidant [Ru(bipy)3]3+ (eqn. B9.1), which has been obtained by a variety of methods - chemical, photochemical and electrochemical oxidation including in situ electrogenerated chemiluminescence. Each of these generation methods has been discussed in a comprehensive review by Barnett and co-workers.[1] Chemical generation of [Ru(bpy)3]3+ has been achieved by a range of reagents such as cerium(IV) sulphate, lead dioxide and potassium permanganate.
The chemiluminescence reactions between primary, secondary or tertiary amines and [Ru(bpy)3]2+are very sensitive and have been widely applied to the determination of various analytes containing an amine functionality. The chemistry of electrogenerated chemiluminescence activity of tertiary amines with [Ru(bpy)3]2+ and their chemiluminescence reaction mechanism have been reviewed by Knight and Greenway[2] and that of the chemiluminescence reaction between secondary amine and tertiary amine arising from hydrolyzed and unhydrolyzed β-lactam antibiotics, respectively, has been reported by Liang et al.. More recently, there have been several reports dealing with the detection and determination of drugs by using the [Ru(bpy)3]2+/potassium permanganate system. These included tetracyclines, cinnamic acid, enalapril maleate and metoclopramide hydrochloride
References
[edit | edit source]
Oxygen radicals
B10. Oxygen radicals
[edit | edit source]Modest chemiluminescence occurs when solutions of iron(II) ions or titanium(III) ions are added to carbonate buffer at alkaline pH,[1] the intensity increasing with the metal ion concentration. This occurs even in solutions that have been deaerated with nitrogen. Surprisingly, the chemiluminescence of deaerated solutions sometimes exceeds that observed in oxygenated solutions. If luminol is also present the intensity of the chemiluminescence is increased (by a factor of about 100 for 1 x 10−5 mol dm−3 luminol), even though the only oxidant present is dissolved oxygen. The presence of the fluorophore rhodamine B also increases the chemiluminescence intensity, but the enhanced chemiluminescence is always more intense in oxygenated solutions. It is possible that other metal ions of low oxidation number, having reducing properties, will also induce this effect. Cobalt(II) ions or copper(II) ions have been shown to give rise to chemiluminescence when added to alkaline solutions of luminol with no added oxidant.
The phenomenon can be rationalized in terms of the well-established chemistry of single electron oxidation of iron(II) in solution.[2]
(B10.1) Fe2+ + O2 → Fe3+ + O2•―
(B10.2) Fe2+ + O2•― + H+ → Fe3+ + HO2―; followed by HO2― + H+ → H2O2
(B10.3) Fe2+ + H2O2 → Fe3+ + HO• + HO―
(B10.4) Fe2+ + HO• → Fe3+ + HO―
The oxygen radicals so produced are the effective chemiluminescence reagent. Radicals can recombine to generate products in excited states, which emit light. The surprising result that chemiluminescence is more intense when the solutions are de-aerated may be due to the more rapid oxidation of iron(II) in oxygenated solutions, leading to initially high concentrations of radicals which fall rapidly as they are converted to hydroxyl ions, so that transient high chemiluminescence would occur too soon to be detected in the flow system used. Luminol chemiluminescence initiated by iron(II) is no doubt due to primary oxidation by hydroxyl radicals (alone or in association with Fe2+), followed by secondary oxidation by superoxide. The light emission occurring when reductants are added to an alkaline solution of luminol and potassium ferricyanide is a special case of this reaction.
The iron(II)-luminol reaction has been applied to the determination of iron(II) in water under natural conditions at nanomolar and micromolar concentrations.[3] It is claimed to be a better assay than ultraviolet/visible spectrophotometry, titrimetry or polarography, having the advantages of high sensitivity, extreme rapidity and simplicity of operation, low cost and avoiding pre-treatment of the sample. It distinguishes iron(II) from iron(III) and can be adapted to measure total iron. Titanium(III)-luminol chemiluminescence has been applied to the determination of titanium(IV) which was converted to titanium(III) by on-line reduction. Fenton’s reagent, a mixture of aqueous iron(II) ions and hydrogen peroxide, has been used to promote chemiluminescence by oxidation. An example is the determination of amines and amino-acids after derivatization to Schiff bases.[4] A selective determination of adrenaline has also been reported.
References
[edit | edit source]
Sulfites and persulfates
B11. Sulfites and persulfates
[edit | edit source]Sulfite is a well-known reductant. Oxidation of aqueous sulfur dioxide by acidified permanganate, cerium(IV) or hydrogen peroxide is feebly chemiluminescent;[1] exploitation of the weak chemiluminescence improved the detectivity of atmospheric sulfur dioxide by a factor of 50. A proposed mechanism comprised an initial oxidation of HSO3― to S2O62― , which then disproportionates to SO42― and excited SO2, which emits visible light. Sulfites undergo an addition reaction with carbonyl compounds and addition of cyclohexanone to protect sulfite solutions against atmospheric oxidation led to the observation that this, at appropriate concentrations, enhanced the oxidative chemiluminescence. Light emission is also sensitized by other cyclohexyl compounds. Paulls and Townshend have suggested that the enhancement depends on β-sultine formation and have shown that the phenomenon occurs generally with higher cycloalkyl compounds, the optimum ring size being nine.
Fused cycloalkane rings also enhance the oxidative chemiluminescence of sulfites and this has given rise to a number of assays for steroids. Thus, a range of corticosteroid drugs have been determined by enhancing the chemiluminescence of sulfite oxidized by cerium(IV). Steroid hormones enhance the chemiluminescence of sulfite oxidized by bromate or by cerium(IV) and an assay based on this effect has been reported. In addition, bile acids sensitize the light emission accompanying the oxidation of sulfites by a variety of oxidants (Ce4+, MnO4―, BrO3― or Cr2O72― and these reactions have been applied analytically.
There is evidence that the chemiluminescence of the permanganate-sulfite reaction has the same emitter as any other permanganate oxidation and the red emission from this persists in the presence of fluorophores as a major contributor to total light output.[2] The cerium(IV)-sulfite reaction does not have any effect on the chemiluminescence spectrum in the presence of fluorophores. The spectra emitted by bromate and dichromate oxidations have not been studied. It is therefore still possible that the chemiluminescence reactions with sulfite might have the mechanism described above, leading to emission from excited sulfur dioxide. There have been persistent reports of emission from the permanganate-sulfite reaction at lower wavelength than can satisfactorily be ascribed to manganese(II) phosphorescence – the usual mechanism – but these can be explained at least partly by the use of spectroscopic data that has not been corrected for the variation in sensitivity of the detector at different wavelengths.
Whereas sulfites promote chemiluminescence due to their reducing properties, persulfates act as oxidizing agents in chemiluminescent reactions. These do not have sulfur in a higher oxidation state than normal sulfates; rather, they contain peroxide units, where two catenated oxygen atoms take the places of two separate oxygen atoms, one in each of the two linked sulfate groups; these oxygen atoms are in oxidation state −I. Chemiluminescence has been reported from persulfates, both by electrochemical reduction at magnesium, silver or platinum electrodes and by thermal decomposition at the surface of magnesium.[3] The light-emitting species in each case are reported to be oxygen radical ions, O•―, and excited peroxide ions, O22―, arising respectively by deprotonation of hydroxyl radicals, HO•, or of hydrogen peroxide or hydroperoxide radicals, HO2•. Persulfates are also used as oxidants in luminol chemiluminescence and as ancillary oxidants in ruthenium chemiluminescence, where they generate the oxidant [Ru(bipy)3]3+ (see eqn. B9.1).
References
[edit | edit source]
Hypohalites and halates
B12. Hypohalites and halates
[edit | edit source]Chemiluminescence reactions involving hypohalites and related oxidants have been exploited for a wide variety[1] of analytical applications, primarily for the determination of free chlorine, halides and a variety of compounds in pharmaceutical preparations and natural waters. Proposed mechanisms of the light-producing pathways are insufficiently supported by spectroscopic evidence but, where emission spectra are known, large differences show that numerous different emitters are involved. A deeper understanding of the light-producing pathways and hence the relationship between analyte structure and chemiluminescence intensity is required.
Two examples of the use of halates in chemiluminescence will now be mentioned. A novel flow-injection system for the determination of formaldehyde has been described.[2] It is based on a strong enhancement by formaldehyde of the weak emission from the reaction between potassium bromate and rhodamine 6G in sulfuric acid. The method has been applied to determine formaldehyde in the air samples and a possible mechanism has been proposed.
The oxidation reaction between periodate and polyhydroxyl compounds has also been studied.[3] A strong emission, especially in the presence of carbonate, is observed when the reaction takes place in a strongly alkaline solution (but not in acidic or neutral solution) without any other chemiluminescence reagent. Background and chemiluminescence signals of the sample are enhanced by oxygen and decreased by nitrogen. The chemiluminescence spectrum shows two main bands (at 436-446 nm and 471-478 nm). Based on these, a possible chemiluminescence mechanism has been proposed. Two emitters contribute to the chemiluminescence background, singlet oxygen and carbonate radicals.
The addition of polyhydroxyl compounds or hydrogen peroxide causes enhancement of the chemiluminescence signal. This reaction system has been developed as a flow injection assay for hydrogen peroxide, pyrogallol, and α-thioglycerol. The ions involved in the reaction - periodate, carbonate and hydroxyl - can be immobilized on a strongly basic anion-exchange resin and highly sensitive chemiluminescence flow sensors for each analyte have been assembled.
References
[edit | edit source]
Micellar enhancement
C. Enhancement of Chemiluminescence
[edit | edit source]C1. Micellar enhancement
[edit | edit source]Well-defined mechanistic principles have emerged to rationalize micellar enhancement of chemiluminescence. The review of Lin and Yamada[1] focuses on how micelles may be used to improve chemiluminescence signals by changes that affect the reaction rate. These occur in the microenvironment (i.e. polarity, viscosity and/or acidity, etc.), in the chemical and photophysical pathway and in the solubilization, concentration and organization of the solute/reactant. We shall now use these principles as a framework for discussing this work and it will become clear that they are highly inter-related rather than mutually exclusive.[2]
There follow examples of micellar enhancement which have been explained by changes in the microenvironment. In the interaction of sulfite groups in drugs with dissolved oxygen in presence of acidic rhodamine 6G, the surfactant Tween 60 can enhance chemiluminescence by 200%, attributable to a microenvironment that leads to an increase in the fluorescence quantum yield of rhodamine 6G and prevents quenching by oxygen. Sensitization of IO3/H2O2 chemiluminescence in the presence of various surfactants at various concentrations has been explained by changes in the microenvironment rather than by solubilization, electrostatic effects or changes in pH. In the chemiluminescence reaction of luminol with hypochlorite in cetyltrimethylammonium chloride (CTAC) micelles, the light reaction in micellar media results in chemiexcitation yields which are higher than those in the corresponding homogeneous aqueous media due to the less polar microenvironment of the micellar stern region but the actual chemiluminescence quantum yields are lower due to quenching, both chemical and photophysical.
In some cases there is evidence of changes in chemical or photophysical pathways or rates of particular reactions. In the system of lucigenin reduced by fructose, glucose, ascorbic acid or uric acid, the cationic surfactant cetyltrimethylammonium hydroxide (CTAOH) increases the chemiluminescence intensity better than cetyltrimethylammonium bromide (CTAB) due to the superiority of CTAOH in micellar catalysis of the rate-limiting step of the lucigenin-reductant reaction. In permanganate chemiluminescence for the analysis of uric acid in the presence of octylphenyl polyglycol ether, there is an alteration in the local microenvironment allowing the solute to associate with the micellar system and this affects various photophysical rate processes. A small amount of surfactant added to the luminol-gold(III)-hydroxyquinoline system, can stabilize gold(III) in aqueous solution, accelerate the reaction rate and hence increase chemiluminescence intensity. The surfactant Triton X-100 can accelerate the chemiluminescence reaction between colloidal manganese dioxide (MnO2) and formic acid in perchloric acid but CTAB or sodium dodecyl sulfate (SDS) cannot.
Sometimes the micelles have their enhancing effect by changing the local concentrations and organization of the reactants. The determination of iron(II) and total iron by the effect on the luminol/hydrogen peroxide system is enhanced by tetradecyltrimethylammonium bromide (TTAB) in the presence of citric acid. An iron(II)-citric acid anion complex is formed and concentrated at the surface of the cationic micelle. This then reacts with hydrogen peroxide at that surface, increasing the rate of the chemiluminescence reaction. The effect of cationic surfactant on the copper-catalysed chemiluminescence of 1,10-phenanthroline with hydrogen peroxide is that 1,10-phenanthroline concentrates in the centre of the micelles, but superoxide anion radicals are attracted to the surface where the reaction occurs more easily.
Some cases of micellar enhancement are explained by facilitation of energy transfer. Greenway et al.[3] found that a non-ionic surfactant helps to overcome the pH imbalance between codeine (in acetate buffer) and Ru(bipy33+ (in sulfuric acid and Triton X-100). The reacting species are enclosed within a micelle which enabled easier energy transfer. CTAB micellar complexes enhance the signal in the presence of fluorescein in the luminol-hydrogen peroxide system. The effect on energy transfer arises because the aminophthalate anion energy donors and the fluorescein anion acceptors will be located at distances approximately corresponding to diameter of micelle (1-3 nm). Since, the transfer of electron excitation energy in solutions can be realized up to a distance of 7-10 nm (Förster mechanism, see chapter C2) (ADD LINK), the concentration of both species in the micelle is very effective for energy transfer. The same explanation applies to the chemiluminescence reactions of luminol and its related compounds in the presence of CTAC, which is also enhanced by intramicellar transfer of electronic excitation energy. Intramicellar processes of energy transfer can easily be modified by altering surfactant concentration and optimized in order to reach maximum conversion of chemical energy to emitted light. The procedure is generally applicable, the effectiveness varying a little with different chemiluminescence reactions, acceptors of electron excitation energy, catalysts and surfactant enhancers.
References
[edit | edit source]
Dye enhancement
C2. Dye enhancement
[edit | edit source]Chemiluminescence is often very weak and to use it, or even to investigate it, it is necessary to enhance it. One way to do this is to use fluorescent dyes. So it is necessary to find a link between the properties of the dye and the degree of enhancement achieved. One key property is the fluorescence quantum yield of the dye; this must be greater than the chemiluminescence quantum yield of the original emitter.
There are two processes by which a luminescent signal can be enhanced, depending on the distance separating the emitting molecule (the energy donor) from the dye molecule (the energy acceptor). The Dexter mechanism applies at very short separation distances, for example when molecules collide. This very close approach allows the excited state donor to exchange a high energy electron for one of lower energy, thus returning to the ground state. The ground state acceptor molecule loses the low energy electron and gains one of higher energy, thus entering an excited state. The rate of energy transfer depends on the concentration of acceptor molecules.
For molecules that are further apart (up to 7-10 nm), the Förster mechanism applies. This involves direct transfer of energy from donor to acceptor, rather as a radio antenna transmits energy to a receiver. The relationship between the rate constant of energy transfer (kET) and the separation distance (R) is given by:
kET = (1/τD)(R0/R)6
where τD is lifetime of the excited state of the donor molecule and R0 is the critical separation constant. The actual rate of energy transfer depends on the rate constant and on the concentrations of donor and acceptor molecules. Also important is the extent of overlap between the emission band of the donor and the absorption bands of the acceptor. This is greatest when the maximum emission wavelength of the donor is close to the maximum absorption wavelength of the acceptor, but it also depends on the shapes of the bands and on the bandwidths. The molecular structure of the donor and acceptor molecules determine the probability of energy transfer.
The chemically initiated electron exchange luminescence model (CIEEL), proposed to explain peroxy-oxalate chemiluminescence[1] (see chapter B5) (ADD LINK) may sometimes apply to dye enhancement. It has been observed that higher and slimmer chemiluminescence signals, implying a more rapid rate of the light emitting reaction, are obtained when cerium(IV) and rhodamine 6G are premixed before the injection of the sample. Oxidation of rhodamine 6G by cerium(IV) would certainly form excited state cerium(III), but this would add to the baseline and blank signals as well as to the sample peaks; it would therefore not explain the observed premixing effect. It appears that instead an oxidation product of rhodamine 6G is responsible, for this has an opportunity to react with the sample, leading to specifically enhanced analyte signals. There was no advantage in increasing the time available for the pre-oxidation of rhodamine 6G, so it seems likely that the active product is formed on first contact and could be an intermediate formed by single electron transfer. The electron is transferred from this initial oxidation product to the analyte, reducing it back to rhodamine 6G in an excited state, giving us the analyte (A) oxidation:
Rh6G ― e― → Rh6G•+
Rh6G•+ + A → Rh6G* + A•+
Emission from excited rhodamine 6G would occur as before. If (as is plausible) this single stage formation of excited rhodamine 6G goes further and faster than the two stages of analyte oxidation by cerium(IV) followed by energy transfer from excited cerium(III) to rhodamine 6G, it would explain the higher and slimmer analyte peaks that were observed.
References
[edit | edit source]- ↑ Kwakman P J M and Brinkman U A Th, Anal. Chim. Acta, 1992, 266, 175.
Enhancement by ultrasound
C3. Enhancement by ultrasound
[edit | edit source]A novel ultrasonic flow injection chemiluminescence (FI-CL) manifold for determining hydrogen peroxide (H2O2) has been designed.[1] Chemiluminescence obtained from the luminol-H22-cobalt (II) reaction was enhanced by applying 120 W of ultrasound for a period of 4 s to the reaction coil in the FI-CL system and this enhancement was verified by comparison with an identical manifold without ultrasound. The method was applied to the determination of trace amounts of H2O2 in purified water and natural water samples without any special pre-treatments.
It is well-known that alkaline solutions of luminol emit light when subject to ultrasound of sufficient intensity to produce acoustic cavitation. Light emission is believed to occur through a process of oxidative chemiluminescence involving sonochemically generated HO·. The cyclic pressure variations associated with the propagation of ultrasound waves in aqueous solution are known to result in the growth and periodic collapse of microscopic cavitation bubbles filled with gas and/or vapour[2][40]. Furthermore, it has been shown that extremely high local temperatures and pressures may be generated during the collapse or implosion of such bubbles. Consequently, it is generally accepted that it is within the cavitation bubble, or the layer of solution immediately contacting the cavitation bubble, that the sonochemical effects take place.
Luminol chemiluminescence has been described in section B1 (ADD LINK). Light emission from the reaction between luminol and hydrogen peroxide can be induced by the presence of cobalt(II) at concentrations low enough to be regarded as catalytic. The effect of ultrasound on hydrogen peroxide is to produce hydroxyl radicals by homolytic fission of the O―O bond:
H2O2 → 2HO•
Hydroxyl radicals in aqueous solution are short-lived. The consumption of these radicals by recombination is very rapid and attenuates the ultrasound enhancement:
2HO• → H2O2
Because of this, the concentration of hydrogen peroxide soon greatly exceeds that of hydroxyl radical, even if the radicals are initially produced in high yield. There is then a greater probability that radicals will instead react with hydrogen peroxide molecules, forming superoxide:
HO• + H2O2 → O2•─ + H3O+
As a result, the effect of sonication is the production in the sample of superoxide rather than hydroxyl radicals. The hydroxyl radicals initially formed would have reacted with luminol to initiate the light-emitting pathway but the primary oxidation of luminol by superoxide is negligible. Instead, when the sample merges with luminol/buffer/cobalt, the effect of this enhanced superoxide concentration is to increase the concentration of the hydroperoxide intermediate, enhancing the light emitting pathway where it has already been initiated by cobalt/hydrogen peroxide; this leads to a fivefold improvement in the detection limit.
The practical implementation of this ultrasound enhancement proved to be exacting. Small changes in the FIA manifold were found to have a considerable effect on the chemiluminescence intensity. In spite of this it was found possible to optimise a range of relevant variables. Some variables were concerned with the arrangements for administering a dose of ultrasound energy to the sample as it flowed through a coil immersed in the sonication bath. To achieve this, the coil had to be long enough to contain the sample all the time that sonication was occurring, but not so long that the enhancement of the chemiluminescence signal would be abolished either by dispersion of the sample into the carrier or by decay of the short-lived radicals generated by sonication. The optimum distances between the water surface and the probe tip and between the probe tip and the upper edge of the sonication coil correspond closely to the conditions for the establishment of standing waves in the sonic bath.
Cavitation when present is the predominant mechanism of acoustic energy absorption as well as providing the collapsing bubbles that are the sites of the sonochemical reactions. Absorption by bubbles is so effective that they provide a shielding effect and so could explain the difficulty in predicting the effect of small changes in the position of the coil within the sonication bath. It was necessary to vary the sonication arrangements in order to optimise them, but operational analytical applications of ultrasound enhancement would be more easily carried out using fixed sonication arrangements in a permanent and purpose-designed apparatus
References
[edit | edit source]
Detection of chemiluminescence
D. Instrumentation
[edit | edit source]D1. Detection of chemiluminescence
[edit | edit source]The detector of choice for chemiluminescence is the photomultiplier tube, a development of the vacuum phototube that permits considerable amplification of the signal. Figure D1.1 shows how the photomultiplier works. The surface of the cathode supports a photoemissive layer that ejects electrons in direct proportion to the intensity of the incident light; several electrons are emitted for each photon and are attracted towards a positively-charged dynode. When the electron beam meets the dynode several electrons (E in figure D1.1) are ejected for each incident electron and these are attracted to a second dynode at a higher positive potential. This process is repeated along a series of dynodes, the intensity of the electron beam increasing continually until when it reaches the anode (at the greatest positive potential) there are over a million electrons for each photon incident at the cathode. The resulting current can be amplified electronically. In the absence of light, the photomultiplier generates a dark current, chiefly due to thermal emission. Thermal dark currents can be eliminated by cooling to ─30 °C.

Figure D1.1 – Principle of the photomultiplier (see text).
Diodes are also used for chemiluminescence detection,[1] especially in low-cost applications. Photographic detection was also used in very early work.[2]
References
[edit | edit source]
Flow injection analysis (FIA)
D2. Flow injection analysis (FIA)
[edit | edit source]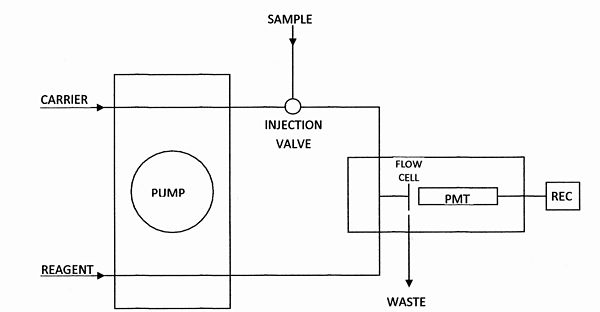
Figure D2.1 – A flow injection manifold for measuring chemiluminescence (PMT = photomultiplier tube; REC = recorder).
Batch techniques for measuring the intensity of chemiluminescence are sometimes used, some of which incorporate automation to improve sample throughput,[1] but flow methods are applied much more often. A suitable flow injection manifold[2] is shown in figure D2.1. Flow injection manifolds are constructed from polytetrafluoroethylene (PTFE) tubing to contain the sample while it is chemically or physically modified prior to detection. Liquid is usually transported from reservoirs by means of a peristaltic pump with suitable tubing. An accurately measured volume of sample is reproducibly introduced into a carrier stream by means of a rotary injection valve. The detector is connected to some means of data-storage. The signal depends on the rate of the reaction producing it and on flow-rate, tubing dimensions, reagent addition order and flow-cell volume, which should be large enough to ensure that a high proportion of the total emission enters the detector; optimisation will favour conditions that lead to emission occurring during the passage of the sample through the flow-cell. The flow-cell should be so positioned as to make this possible, e.g., directly in front of the window of a photomultiplier and in a box that excludes ambient light. FIA has important advantages over batch methods. It makes use of simple and relatively inexpensive apparatus, which is readily miniaturised and has great potential for adaptation and modification. Easy operation and high sampling rates are possible.
References
[edit | edit source]
Sequential injection analysis (SIA): lab on a valve
D3. Sequential injection analysis (SIA):lab on a valve
[edit | edit source]SIA, like FIA, is based on reproducible sample handling and controlled dispersion of sample and reagents into a carrier stream. Unlike FIA, it makes use of a computer-controlled multiposition valve and pump, usually peristaltic and operated synchronously with the valve.

Figure D3.1 – A sequential injection manifold suitable for the determination of morphine by acidified potassium permanganate chemiluminescence.
Morphine is solvent-extracted from opium poppies Papaver somniferum on an industrial scale. Barnett et al. have used SIA to determine the drug in aqueous and non-aqueous process streams, with chemiluminescence detection involving oxidation with acidified potassium permanganate in the presence of sodium hexametaphosphate. Figure D3.1 shows a suitable SIA manifold for carrying out this determination. The process streams contain several related alkaloids and a range of other organic compounds as well as both dissolved and suspended solids. It is a good indication of the effectiveness of SIA-chemiluminescence that in these conditions the results correlated well with high performance liquid chromatography, a standard methodology that suffers the defect a much lower sample throughput.
In SIA, sample and reagents are aspirated into the holding coil by operating the pump in reverse so that carrier is returned to the reservoir. Restoration of forward pumping is synchronised with the opening of the valve port leading to the detector. The flow reversal leads to a mixing of the stack of sample and reagent zones to form a product zone which is transported to the detector. The pump tubing comes into contact only with the carrier, the samples and reagents being aspirated (instead of pumped) into the holding coil. This is a very useful characteristic of SIA when using samples /reagents that would attack PVC pump tubing, such as those containing non-aqueous solvents.
Lab on a chip
D4. Lab on a chip
[edit | edit source]Micro total analytical systems (also called “chips”) are miniaturized microfluidic devices, fabricated from a variety of materials within which channels are constructed for the transport of samples and reagents. The small size minimizes the consumption of reagents, reduces manufacturing costs and increases the possibilities for automation. Miniaturization of detectors, however, leads to problems due to the reduced volume of liquid in the detector and to difficulties inherent in scaling down the size of the particular detector. One solution is to interface the chip with a macro-scale detector such as a photomultiplier tube; this is called the “off-chip” approach. This can be achieved, for example, by using optical fibres to carry light from the chip to the detector. An alternative solution – the “on-chip” approach - is to assemble a compact version of the detector and integrate this on the chip with the rest of the analytical system.[1]
Chemiluminescence detection offers high sensitivity, low detection limits and instrumental simplicity but requires a relatively complex manifold on the microchip, the details depending on the chemiluminescence reaction system being used; for example, a Y-shaped channel junction works best when using peroxide-luminol chemiluminescence. Reagent is delivered by a micropump. The chip design must ensure that a high proportion of the emitted light enters the off-chip photomultiplier; this frequently involves coupling with an optical fibre. Such an arrangement typically achieves micromolar detection limits and has been used for a range of analytes including catechol, dopamine, amino-acids, cytochrome c and myoglobin as well as the determination of chip-separated chromium(III), cobalt(II) and copper(II). Horseradish peroxidise can be determined at sub-nanomolar levels. Micromolar concentrations of ATP (adenosine triphosphate) can be measured by means of luciferin-luciferase bioluminescence. The effect of antioxidants has been measured using a microfluidic system incorporating peroxy-oxalate chemiluminescence, by injecting the antioxidants into the hydrogen peroxide stream. The method is simple and rapid and excellent analytical performance is obtained in terms of sensitivity, dynamic range and precision. Electrochemiluminescence detection has been applied for microchip separations using electrodes installed during fabrication.
Photodiodes have been fabricated into chips at the bottoms of the microfluidic channels and have been used for on-chip chemiluminescence detection of DNA produced by the polymerase chain reaction and separated on the same chip by capillary electrophoresis. These devices have been used also to detect luminol chemiluminescence for the micromolar determination of hydrogen peroxide generated by the oxidation of glucose with glucose oxidase. Thin-film organic photodiodes can be fabricated by vacuum deposition and integrated into chips. Copper-phthalocyanine-fullerene small molecule diodes have high quantum efficiency and have been used to determine hydrogen peroxide by peroxy-oxalate chemiluminescence. Another example has been used for hydrogen peroxide determination by luminol chemiluminescence.
References
[edit | edit source]- ↑ Kuswandi B, Nuriman, Huskens J and Verboom W, Anal. Chim. Acta, 2007, 601, 141-155.
Chemiluminescence sensors
D5. Chemiluminescence sensors
[edit | edit source]Chemiluminescence has the advantage of lower background emission than fluorescence, avoiding noise caused by light scattering. However, because chemiluminescence reagents are irreversibly consumed, chemiluminescence sensors have shorter lifetimes than fluorescence sensors and their signals have a tendency to drift downwards due to consumption, migration and breakdown of reagents. Reagent immobilization onto suitable substrates plays an important role in the development of chemiluminescence sensors. Selectivity and sensitivity as well as lifetime of chemiluminescence sensors depends on the choice of reagent and substrate and on the method of immobilization.[1]
Chemiluminescence reagents are typically aqueous solutions of ions and so can be immobilized by convenient procedures onto ion exchange resins, giving high surface coverage, and released quantitatively by appropriate eluents. Analytes can also react directly with immobilized reagents. These properties have been widely used to prepare chemiluminescence sensors containing immobilized luminol or other reagents, which typically would be packed into a flow cell positioned in front of the window of a photomultiplier. “Bleeding” columns of anion/cation exchange resins with co-immobilized luminol and metal ions such as Co2+, Cu2+ or [Fe(CN)6]3– can detect and measure analytes such as hydrogen peroxide, though this arrangement causes unnecessary dilution of samples and reagents, which impairs detectivity. Immobilized tris-(2, 2/-bipyridyl)ruthenium(II) can be regenerated from tris-(2, 2/-bipyridyl)ruthenium(III) and can be used for at least six months.
Immobilization of enzymes can be used to produce highly active and selective chemiluminescence sensors from which enzyme is not consumed, though their operational stability is limited. Encapsulation of reagents in sol-gel silica involves little or no structural alteration and is very suitable for chemiluminescence sensors because of its optical transparency and chemical stability. For example, encapsulated horseradish peroxidase displays high activity and long life, as does sol-gel immobilized haemoglobin. Chemiluminescence sensors constructed from plant and animal tissues have advantages of cost, activity, stability and lifetime; examples are soyabean tissue in sensors for urea and spinach tissue in sensors for glycolic acid.
Molecular imprinted polymers have been found to be very useful materials for fabrication of chemiluminescence sensors, both as molecular recognition agents and as chemiluminescence reaction media. Analytes that can be successfully detected in this way include 1,10-phenanthroline and dansylated amino-acids. Metal oxide particles can sometimes be entrapped onto membranes or in columns, including chemiluminescence flow cells. This affords a simple fabrication method producing long-lived sensors. Manganese dioxide has been immobilized in this way on sponge rubber for the assay of the drug, alangin, using manganese(IV) chemiluminescence.
Chemiluminescence has been detected from surface reactions on nanoparticles, opening up the possibility of chemiluminescence nanosensors of good stability and durability. Coumarin C343, a fluorescent dye, has been conjugated to silica nanoparticles entrapped in sol-gel silica to produce nanosensors capable of enhancing the weak chemiluminescence associated with lipid peroxidation.[2]
References
[edit | edit source]
Chemiluminescence imaging
D6. Chemiluminescence imaging
[edit | edit source]Chemiluminescence imaging combines the sensitive detection of chemiluminescence with the ability to locate and quantify the light emission, but above all it massively provides parallel determinations of the analyte. A digital image is made up of thousands of pixels, each generated by an independent sensor, detecting and measuring the light that falls on it. This enables simultaneous measurement of multiple samples or analytes for high throughput screening.
Chemiluminescence imaging microscopy detects labelled probes more simply and more accurately than does fluorescence. It could become an important tool for rapid, early diagnosis of a wide range of diseases. Whole animal in vivo chemiluminescence imaging makes possible real-time monitoring of pathological and biological phenomena and we may anticipate important advances of great impact in drug discovery, biotechnology and medicine.[1]
D6a. Imaging sensors
[edit | edit source]The last twenty years have witnessed a steady improvement in our ability to form images from analytical signals. Imaging makes use of the high sensitivity and specificity, low background and wide dynamic range of chemiluminescence to quantitate and localize analytes down to the level at which this can be achieved by emission of single photons. Early systems consisted of a low-light vacuum tube device[2] connected to an optical microscope. CCD (charge coupled device) and CMOS (complementary metal oxide semiconductor) image sensors make use of different technologies, both invented in the late 1960s and 1970s, for capturing images digitally. Both types of imager convert light into electrical charge and process it into electronic signals, as in digital cameras. Each has characteristic strengths and weaknesses. CCD technology is in most respects the equal of CMOS. Costs are similar.
a(i). Charge coupled devices (CCD)
[edit | edit source]It is central to chemiluminescence imaging that when the spatial distribution of the analyte is critical, a luminograph must be produced to adequately express the data. This can be done by a CCD, which must have a high light-collection efficiency. A CCD converts optical brightness into electrical amplitude signals. CCDs are arrays of semiconductor gates formed on an integrated circuit (IC) or chip. The gates individually collect, temporarily store and transfer charge, which represents a picture element or pixel of an image. When light falls on a CCD sensor, a small electrical charge is generated photoelectrically for each pixel; each charge is converted to voltage and output as an analogue signal, which can be converted to digital information by additional circuitry. All of the pixel can be devoted to light capture. A CCD camera, such as a digital camera includes a CCD imager IC located in the focal plane of the optical system and control circuits mounted on a printed assembly. Image data captured is stored in a storage medium such as a compact flash memory or an IC memory card and can be displayed on a monitor such as a liquid crystal display (LCD). CCDs have traditionally provided the highest image quality (as measured by quantum efficiency and noise). An intensified CCD (ICCD) camera is optically connected to an image intensifier. In an image intensifier, the photons which are coming from the light source fall onto the photocathode, thereby generating photoelectrons. The photoelectrons are accelerated towards a micro-channel plate (MCP) by a voltage applied between photocathode and MCP. The electrons are multiplied by the MCP and accelerated towards a phosphor screen, which converts them back to photons that are guided to the CCD by an optical fibre or lens. ICCD cameras permit high frame rates and real-time visualization, the limitation being the increased noise produced by amplification. Non-intensified slow scan CCD cameras, cooled to reduce thermal noise, permit integration of the signal over a relatively long time and are suitable for steady-state signals.
a(ii). Complementary metal oxide semiconductor (CMOS) chips
[edit | edit source]CMOS image sensors have emerged as an alternative to CCD sensors. They consist of an integrated circuit (IC) containing an array of pixel sensors, each pixel containing a photodetector, an amplifier and additional circuitry to convert the charge (which represents light intensity) to a voltage. Amplification, noise-correction, and digitization often also occur on-chip. These other functions increase the design complexity and reduce the area available for light capture. Unlike CCD sensors, each pixel is doing its own conversion, so uniformity is lower, which affects image quality. But the chip requires less off-chip circuitry. The phrase "metal oxide semiconductor" implies transistor structure having a metal gate electrode on top of an oxide insulator, which in turn is on top of a semiconductor. CMOS sensors complement every n-type transistor with a p-type, connecting the pairs of gates and drains. Because ideally no current flows except when the inputs to the gates are being switched, this greatly reduces power consumption and avoids overheating, which is a major concern in all ICs. CMOS can potentially be implemented with fewer components, uses less power and provides faster readout than CCDs. CMOS imagers also offer greater integration (more functions on the chip) and smaller size.
It is possible to use a CMOS sensor chip as a microscale contact imager and quantitative photometer for chemiluminescence assays. The applicability has been investigated for chemiluminescence detection of ATP by its reaction with a proprietary reagent in 1 mm diameter wells fabricated on a glass cover-slip placed directly onto the imaging sensor.[3] Ambient light was excluded. For each well, chemiluminescence intensity was averaged over a 1 x 100 pixel region of interest and integrated over a 200 ms exposure. It correlated well with the ATP concentrations over a range of 0.1-1 mmol dm−3. The detectivity (<1 nmol amounts of ATP) is not as fine as can be obtained with a much more expensive CCD camera, but is susceptible to improvement. CMOS chips are suitable for droplet microfluidics or lab-on-a chip devices when the cost of the assay system is a factor that must be optimized, such as in “point-of-need” assays or diagnostics.
a(iii). Photon counting
[edit | edit source]Cameras suitable for luminescence imaging should be able to form an image at a brightness of 10−6 lux (1 lux = 1/621 W m−3 of 550 nm light). The most sensitive detect single photons with an efficiency of about 20% and have an average noise level of 2 x 10−11 lux = 8 photons s−1 cm−2. Available ultra-low light imaging systems include the imaging photon detector (IPD), used in conjunction with a microscope with high numerical aperture (NA) objective lens. A high NA lens collects more light than a low NA lens. The collected light is focussed onto the IPD. Photons are recorded and stored as a list of time and space coordinates created by the IPD processor. Images can be reconstituted as an array of dots over any desired time interval. This allows for continuous recording over any interval; 1 h at 100 photons per second can be stored in 1 Mb of memory (storage as images requires 0.3 Mb per frame). Chemiluminescence images are based on small numbers of photons, especially when exposure time is brief; whereas a ten minute exposure at 100 photons per second can build an image of 60000 photons, a one second exposure provides only 100 photons, so that the image comprises only 100 dots spread across the area of the image[4] A high resolution (up to 1392 x 1040) single photon counting camera system is suitable for extremely low photon-emission applications such as some chemiluminescence applications. The system includes a control unit with data acquisition and image processing software. Frame rates up to 100 Hz can be obtained.
The conceptualization involved in the design of photon counting cameras is well illustrated by DELTA camera; initially designed for astronomy it has advantages for a wide range of high-resolution problems. It is a high sensitivity array detector, which yields the space and time coordinates of photon events at sustained count rates superior to one million per second.[5] It has a flat field, very high resolution (for the prototype: 512 x 591 pixels in space and 2.6 μs in time) and high throughput. Each photon produces an intensified phosphor image which has the same position in a two-dimensional field as the photon. This image is focussed onto three one-dimensional CCDs, which record its position as three coordinates on axes mutually oriented on a plane at angles of 120°. Software converts these to (x, y) coordinates on orthogonal axes and a clock signal adds the time (t) of the event to produce (x, y, t) coordinates, which are listed and stored; artefacts due to excessive tolerance or to simultaneous photon events are also removed from the data.
a(iv). Chemiluminescence imaging systems
[edit | edit source]A high resolution CCD camera (up to about 6.0 Mpixel), cooled to about -70 °C, gives the best quality images, better accuracy, longer exposure times (up to 24 hours), minimal dark noise and enhanced stability. There should be a light-tight dark chamber and a height adjustable sample platform with a numeric counter for exact positioning in specific, repeatable positions. The alternative is an advanced motorised robotic camera which is driven up and down allowing placement very close to the sample, outperforming a standard zoom lens, having a wider field of view, easier and faster operation, and better sensitivity. These benefits are especially useful for faint samples. The image acquisition and analysis software provides comprehensive tools for simple image capture and analysis of gels, plates and membranes as well as colony counting. Images can be enhanced, user preferences defined, reports generated and data exported. Some systems include an overhead white light so that chemiluminescence can be overlaid onto a reflected light image (the so-called “live image”) and combine facilities for fluorescence, chemiluminescence and colorimetric applications.
Not all chemiluminescent reactions are suitable for imaging; the main requirement, especially for imaging microscopy, is micrometre scale localization.[1] Excited species of short half-life are suitable and conditions (especially reactant concentrations) can be optimized to minimize the diffusion of excited products. Glow-type kinetics, arising from the attainment of a steady state, facilitates measurement procedures. Enzyme labels are widely used.
D6b. High throughput screening (HTS)
[edit | edit source]Imaging is very suitable for high-throughput screening. Examples of assays with very high sample throughput include the determination of bioavailable mercury which has been determined in urine using E. coli expression of luciferase under the control of a mercury-inducible promoter. Throughput is more than 5000 samples per hour and the limit of detection is 10-13 mol dm−3. Acetylcholinesterase inhibitors can be assayed using acetylcholinesterase, choline oxidase and horseradish peroxidase (HRP). Kinetic analysis of luminol chemiluminescence is carried out with a throughput of 180-360 samples per hour.
b(i). Miniarrays
[edit | edit source]Imaging with flat field correction lenses can be used to read microtitre plates (up to 4 of 384 wells) faster than by a luminometer, but the latter is, however, more sensitive and has the ability to measure fast, flash-type reactions. Miniarrays of antibody or gene probes can be spotted onto 96-well microtitre plates and assayed with an enzyme-labelled detection reagent and a chemiluminescence substrate. The whole plate is imaged with a CCD camera to measure the light emission from each well. This principle has been applied to a sandwich-type enzyme-linked immunosorbent assay (ELISA) for cytokines and a hybidization-based mRNA assay with up to 16 spots in each well; a 16 x 96 array contains 1536 dots, making possible high-throughput multianalyte assays, using standard plates and their associated sample handling devices.
Oligonucleotide probes specific for human papilloma virus (HPV) genotypes have been used for a multianalyte chemiluminescence imaging assay for the simultaneous determination of up to 7 HPV DNAs. Amplification by the polymerase chain reaction(PCR) in the presence of a digoxigenin-labelled nucleotide (dUTP) is followed by an ELISA using a novel polystyrene microtitre plate having an array of 24 main wells (containing digoxigenin-labelled PCR product) each divided into 7 subwells (containing the immobilized probes). The digoxigenin label was subsequently detected by peroxidase-labelled antibody and a chemiluminescent substrate. Imaging was performed using an ultrasensitive CCD camera.[6] Results were comparable with conventional colorimetric PCR-ELISA.
b(ii). Microarrays
[edit | edit source]Thousands of simultaneous determinations can be made by high-resolution imaging of chemiluminescence at array densities of up to hundreds of spots per square centimetre. Array-based gene expression analysis is a good example. Protein analysis based on antigen-antibody or ligand-receptor interactions is increasingly used in clinical and research work and in drug discovery. As well as their use for protein expression profiling, there are high-throughput protein microarrays that detect up to 35 cytokines. Specific antibodies are spotted onto membranes, which are incubated with the samples and captured analytes are detected by enhanced chemiluminescence with HRP-labelled antibodies and an HRP-substrate. A protein chip for parallel ELISAs of tumour marker allows the discovery of patterns that can increase the sensitivity and specificity of the diagnosis.[7] Immobilized on the chip were 12 monoclonal antibodies against different tumour markers captured by incubating the chip with serum samples. An HRP-conjugated second antibody was used for detection by chemiluminescence imaging. The chip has been successfully applied both for cancer diagnosis and for screening asymptomatic populations at high risk.
b(iii). Small-scale analytical devices
[edit | edit source]Small scale analytical devices use extremely small sample volumes and so need very sensitive detection techniques; chemiluminescence imaging has the high resolution and high sensitivity necessary. Assays include the ELISA determinations of the herbicide 2,4-D in multiple samples using gold-coated surfaces or glass capillaries and of up to ten antibiotics in milk in five minutes: the sample is incubated with mixed monoclonal antibodies followed by detection with an HRP-labelled second antibody and a suitable chemiluminescent substrate. Multiple hybridizations can be performed in a three-dimensional chip incorporating an array of vertical glass channels. Specific gene probes are immobilized on the inner walls of the channels. This strengthens the signal by providing a larger area for probe-immobilization than is available in a two-dimensional microarray. The sample flows through the channels and the analyte is detected by an enzyme-labelled antibody followed by a chemiluminescent substrate. Lateral diffusion of the emitting species is prevented by the walls of the microchannel; this improves resolution of the image. Chemiluminescence imaging of miniaturized analytical devices is also useful for multiplexing (simultaneous quantitation of different analytes or on different samples) by integrating the chemiluminescence over a different target area for each analyte or sample.
b(iv). Documentation of gels and membranes
[edit | edit source]Chemiluminescence imaging detection with CCD cameras can be used for reactions that take place on gels and membranes. This allows intensity measurement over a wide dynamic range and software exists to compute the total emission from particular zones of the image for analytical purposes. Images can be stored on discs or printed out.
Electrophoresis is the movement, and hence the separation, of charged molecules in an electrical field; electrophoresis on polyacrylamide gel (PAGE) is particularly good for separation of molecules at low concentrations. Separated molecules can be transferred onto a nitrocellulose membrane by electroblotting - electrophoresis in a direction at right angles to the gel surface; this is also called western blotting and it is used to detect specific proteins in a sample of tissue homogenate or extract. By similar procedures, Southern blotting identifies particular sequences of DNA within a complex mixture and northern blotting locates RNA. In western blotting, electroblotting is followed by immunostaining, in which particular proteins are identified by labelled antibodies. DNA and RNA can be similarly identified by hybridization with labelled probes.
Dot blot is an immunological technique to detect with antibodies specific proteins in mixtures or in samples such as tissue lysates. It is based on western blotting but there is no separation of the protein on SDS-PAGE. One such assay is the detection of B19 parvovirus. After spotting samples onto a membrane, hybridization with digoxigenin-conjugated DNA probes and treatment with HRP- or AP-labelled anti-digoxigenin antibodies, chemiluminescence imaging gives a limit of detection ten times better than using colorimetry.
Cytochromes can be separated by PAGE with sodium dodecyl sulfate (SDS-PAGE) and transferred to a nitrocellulose membrane; cytochrome c (which contains a catalytic haeme group) is detected by peroxidase-luminol chemiluminescence.[8] CCD imaging results in detection 50 times more sensitive than the 3,3',5,5'-tetramethylbenzidine staining method. A sample of less than 1 ml of a bacterial culture is needed. A similar assay, based on luminol/hydrogen peroxide chemiluminescence with ammonium persulfate enhancement, detects haptoglobin phenotyping after PAGE on a 15 μL sample.[9] Other iron-containing proteins, such as catalase and ferritin, can also be detected. The proposed detection is very fast, compared to traditional staining methods (minutes versus hours).
D6c. Molecularly Imprinted Polymers (MIPs)
[edit | edit source]MIPs have artificial recognition sites with shapes, sizes and functionalities complementary to the analyte, which is thus selected in preference to other closely related structures. They are cheaper and more robust than antibodies, enzymes and biological receptors and can serve when these biomolecules are not available. The recognition sites are fabricated around a suitable template, preferably the analyte itself, which is extracted after polymerization.
Generally, when a template molecule and a functional monomer are mixed in an organic solvent a complex is formed between the template and the monomer through polar interactions. Polymerization with a cross-linker fixes the positions of the polar groups. Removal of the template with a suitable solvent leaves specific recognition sites. The functional monomers are chosen to promote hydrogen bonding with the template to obtain good selectivity and reversibility. Optimum binding occurs when the MIP is exposed to the same conditions as those used in polymerization, because it depends on the shape of the imprinted cavity and on the spatial positioning of the coordinated functional groups. Both of these depend on the conditions and are affected by swelling of the polymer, which can be exploited to achieve fast and controllable release of adsorbed molecules prior to detection.
c(i). Chiral recognition of dansyl-phenylalanine
[edit | edit source]Molecular imprinting of polymers has been linked with chemiluminescence imaging detection to achieve chiral recognition of dansyl derivatives of phenylalanine (Phe).[10] The MIP microspheres were synthesized using precipitation polymerization (which produces uniform microspheres) with dansyl-L-Phe as template and the microspheres were immobilized on microtitre plates (96 wells) using poly(vinyl alcohol) (PVA) as glue. The analyte was selectively adsorbed onto the MIP microspheres. After washing, the bound fraction was quantified using peroxyoxalate chemiluminescence (POCL) analysis, a general method for all fluorescent and fluorescence-labelled analytes, which has a greater quantum yield than most other chemiluminescence systems. In the presence of dansyl-Phe, bis(2,4,6-trichlorophenyl)oxalate reacted with hydrogen peroxide (H2O2) with chemiluminescence emission. The signal was detected and quantified with a highly sensitive cooled CCD. The intensity of the image of each well of the plate was determined using software to sum the intensities of all the pixels making up the spot. Chemiluminescence intensity increases with the proportion of the L-enantiomer in the sample. Chiral composition can thus be determined by comparison of the intensity for the mixture and for pure D- and L- enantiomers at the same concentration. The results show that MIP-based chemiluminescence imaging is useful for quick chiral recognition and, because the method can perform many independent measurements simultaneously in 30 min, high-throughput screening is possible.
c(ii). High throughput detection of dipyridamole
[edit | edit source]A simple, sensitive and specific method has been developed for high throughput detection of the vasodilator dipyridamole.[11] The proposed method is based on a chemiluminescence imaging assay with MIP recognition providing selectivity.
Molecularly imprinted microspheres were prepared using precipitation polymerization with methacrylic acid (MAA) as functional monomer, trimethylolpropane trimethacrylate (TRIM) as the crosslinker and dipyridamole as the template. Non-imprinted polymer (NIP) was prepared without template to use as a control. The microspheres were coated in 96-well microtitre plates using 0.1% PVA as glue. After incubation with the sample, the amount of polymer-bound dipyridamole was determined by POCL. The emitted light was measured with a cooled high-resolution CCD camera. The intensity of the image of each well was determined as in subsection c(i).
Under the optimum conditions, there is a linear relationship between relative chemiluminescence intensity and concentration of dipyridamole ranging from 0.02 to 10 μg ml−1. The detection limit is 0.006 μg ml−1. The method was validated by measuring dipyridamole concentrations in spiked urine samples. High tolerance for a number of normal constituents of urine was demonstrated to be much greater in the presence of MIP rather than NIP. MIP-based chemiluminescence imaging exhibits high selectivity and sensitivity to dipyridamole, combined with high sample throughput and economy (50 μl/well).[12]
D6d. Spatial distribution of targets
[edit | edit source]Target molecules of chemiluminescence imaging include antigens, DNA sequences, enzymes and metabolites. Chemical processes in cells, tissues or whole animals may also be targeted. Methods used include imaging microscopy, immunohistochemistry (IHC), in situ hybridization (ISH); other chemical or enzymatic reactions may also be used. The chemiluminescence image is overlaid onto the visible light image and processed by background subtraction, contrast enhancement, pseudocolour and quantitation over defined areas; absolute quantitation needs reproducible conditions, a calibration system and appropriate sample properties.
d(i). Imaging microscopy
[edit | edit source]Chemiluminescence imaging microscopy uses ordinary microscopes with optimized light collection. Light loss is minimized by having a simple lens coupling system; coverslips are dispensed with. The microscope, or at least the sample, is contained in a dark box to exclude ambient light and has a motorized micrometric stage to permit automatic adjustment. The sample is incubated with the chemiluminescence reagent until a steady-state emission is obtained. The objective lens has the highest numerical aperture (NA) compatible with acceptable focal aberration and depth of field. Dry, rather than oil-immersion, objectives are used and give adequate magnification and spatial resolution for the localization of analytes in single cells or tissue sections;[13] the detection limit for HRP is about 500 molecules/μm2.[14]
Chemiluminescence microscopy has become a standard tool in biomedical research. Photon detectors have been attached to microscopes and allow imaging of chemiluminescent probes and reporter genes in cells and tissues. Photon counting techniques allow days of continuous imaging without creating oversized files. Fluorescence imaging, however, gives better spatial resolution than chemiluminescence imaging and makes multiple determinations easier.
d(ii). Calcium imaging with chemiluminescence microscopy
[edit | edit source]Calcium can be determined in cytosol and in organelles by using the photoprotein aequorin, an intracellular calcium indicator extracted from the jellyfish Aequorea victoria. Natural aequorin consists of a polypeptide, apo-aequorin, covalently bound to a hydrophobic prosthetic group, coelenterazine. The principle of imaging free cytosolic calcium with aequorins[3] is the conformational change of aequorin molecules on calcium binding, causing coelenterazine to be oxidized to coelenteramide with production of carbon dioxide and emission of blue light (466 nm). Aequorin cannot penetrate the plasma membrane of the cell. Microinjection is the method of choice for determining cytosolic calcium in large cells. For small cells, cloning and transfection of the cDNA of apo-aequorin makes microinjection unnecessary, greatly simplifying calcium recording. Genetically expressed apo-aequorin contains no coelenterazine and so does not emit light. It is reconstituted as aequorin by soaking the specimens with coelenterazine. Apo-aequorin can be targeted to specific organelles by incorporating signal translocation sequences in the polypeptide chain.
Aequorin is sensitive and specific, though single cells, containing a low concentration, give feeble chemiluminescence. Intensity is proportional to cell volume and therefore to the cube of the diameter. Small cells present problems because the amount of aequorin is low. In a cell of 10 μm diameter, the resting calcium concentration leads to emission of less than one photon per hour – so fluorescence must be used instead. But elevated calcium concentrations or large numbers of cells can be imaged by chemiluminescence using photon-counting cameras. Natural aequorin accurately measures Ca2+ concentrations in the range 0.5 to 10 μmol dm−3, which is suitable for transient changes but for higher concentrations, a mutant form has been constructed which, by raising its dissociation constant and thus lowering its affinity for calcium, extends the working range up to 100 to 1000 μmol dm−3. It has the advantage over calcium-specific fluorescent probes of permitting real-time measurements over a long period; this is possible as there is no disturbance of the intracellular environment (including Ca2+ buffering capacity) because of the low aequorin concentration (about 5 nmol dm−3) but it has poorer resolution and it is used up rapidly by high calcium concentrations. Aequorin chemiluminescence, however, has an excellent signal to noise ratio and extremely low background noise.
Chemiluminescence calcium imaging using aequorin is the method of choice for exploratory studies, since it is extremely sensitive, can detect a broad range of calcium concentrations. The kinetic order with respect to calcium concentration of the chemiluminescence reaction is 2.1 or higher, which gives inherent contrast enhancement. Unlike fluorescence, it does not require the analyst to make preliminary predictions or assumptions which exclude calcium signals outside the expected range. But it cannot match the high spatial resolution of fluorescence methods. In addition, chemiluminescence microscopy uses a large depth of field and optical sections are not yet possible.
d(iii). Aequorin associated with green fluorescent protein (GFP)
[edit | edit source]In Aequorea victoria, the chemiluminescent calcium-binding protein, aequorin, is associated with GFP. Calcium-sensitive bioluminescent reporter genes have been constructed that fuse GFP and aequorin to increase the quantum yield of calcium-induced bioluminescence.[15] Co-expression of GFP with free aequorin does not have the same effect. The constructs were varied by including different lengths of peptide spacer between the GFP and the aequorin; much more light was emitted in all cases and the constructs were much more stable in cytosol and more sensitive to calcium than recombinant apo-aequorin alone.
Resonance (non-radiative) energy transfer to the GFP chromophore from the excited oxidation product of coelenterazine depends on their relative positions. The peptide spacer is therefore flexible and of variable length. The green:blue ratio (500 nm:450 nm) of the light emitted by different constructs was measured 48 h after the introduction of the reporter genes into the cells (transfection). The green:blue ratio was increased by the covalent attachment of GFP to aequorin and further increased when the linker was added; as the linker was made longer, the wavelength of maximum emission increased and the bandwidth of the spectrum decreased. The efficiency of intramolecular energy transfer is enhanced to a level comparable to that achieved by resonance energy transfer in vivo due to the more favourable configuration made possible by the linker.
Using GFP-aequorin fusions it is possible to detect physiological calcium signals in single cells. Transfection of the cells is followed by aequorin reconstitution with coelenterazine. The result is calcium-induced photon emission detectable with a cooled, ICCD camera, using an integration time of only one second. Cytoplasmic aequorin had previously detected Ca2+ activities only by the use of a photomultiplier, which is more sensitive but lacks any spatial resolution, or by using targeted fluorescence probes, which give a quicker response. The use of the transgenes in which aequorin reports Ca2+ activity while GFP enhances bioluminescence could lead to real time imaging of calcium oscillations in integrated neural circuits in whole animals as well as in specific subcellular compartments. Aequorin and GFP-enhancement probes along with synthetic fluorescent dyes can be targeted to the endoplasmic reticulum (ER),[16] a membrane network within the cytoplasm of cells involved in the synthesis, modification, and transport of cellular materials; this has enabled the role of ER to be clarified.
D6e. Enzyme and metabolite mapping
[edit | edit source]If the sample’s enzyme activity has been preserved and if the sample is in such a condition that the substrate has access to the active site, enzyme activity can be localized by chemiluminescence imaging. The best spatial resolution is obtained by applying a chemiluminescent substrate directly to an enzyme, e.g., alkaline phosphatise can be detected by dioxetane phosphate. Enzyme reactions coupled with chemiluminescence can be used for metabolite mapping but give lower resolution. Metabolites can be determined in shock frozen tissue biopsies at femtomole levels and with micrometre resolution. The tissue is frozen as soon as possible to stop enzyme activity and fix the metabolites. The specimen is then placed on a temperature-controlled microscope stage and the chemiluminescence reagent is added. Emission intensity, recorded as soon as the temperature rises sufficiently, is converted into metabolite concentrations.
e(i). Luciferase-based detection of energy metabolites
[edit | edit source]Measurement[17] of the spatial distribution of metabolites, such as ATP, glucose, and lactate, in rapidly frozen tissue is based on enzymatic reactions that link the metabolites to luciferase with subsequent light emission. Using an array, cryosections are brought into contact with the enzymes in a reproducible way inducing emission of light from the section in proportion to the metabolite concentration, with high spatial resolution. There is a close correlation between the distribution of ATP and cell viability; there are also distribution differences between tumours and normal tissue. ATP, glucose, glycogen and lactate have been determined at microscopic levels and at high spatial resolution in arterial wall cryosections using luciferase-based chemiluminescence imaging,[18] which is a powerful tool to measure energy metabolites. It has been used to quantify local metabolite concentrations in artery rings. Distributions of energy metabolites are heterogeneous under hypoxic in vitro conditions. Diffusion distances for oxygen and nutrients can be long and might make vessels prone to develop local deficiencies in energy metabolism that could contribute to atherogenesis.
e(ii). Expression of luciferases in living cells and organisms
[edit | edit source]Luciferases are enzymes that emit light in the presence of oxygen and a luciferin (ADD LINK). They have been used for real-time, low-light imaging of gene expression; coding sequences have been detected by luciferase-labelled gene probes.[19] These labels include bacterial lux and eukaryotic luciferase luc and ruc genes. Different luciferases differ in the stability/variability of the emitted signal. Luciferases have served as reporters in a number of promoter search and targeted gene expression experiments. Photon-counting CCD imaging of luciferase has been used, for example, to show promoter activity in single pancreatic islet β-cells and regulation of human immunodeficiency virus (HIV) and cytomegalovirus. Luciferase imaging has also been used to trace bacterial and viral infection in vivo and to visualize the proliferation of tumour cells in animal models. Infected cells are readily detectable at an incidence of one in a million cells. Single bacterial cells, whether transformed or naturally luminescent, have also been imaged and variation in expression over time, due to fluctuations in metabolic activity, has been demonstrated. Low-light CCD imaging is in itself a non-invasive technique that is useful for observing (see subsection b(iv). Documentation of gels and membranes ADD LINK) intracellular gene expression and small-scale assays such as in situ hybridization (ISH) as well as for immunoassays, gels and blots [ADD LINK], DNA probes and in vivo imaging (see section D6h, ADD LINK). Slow-scan liquid nitrogen cooled CCD cameras are preferable for high resolution imaging with long exposures, but photon-counting CCD cameras are better for shorter exposure times. Flashing at frequencies greater than 1 Hz can be detected by ICCD cameras.
e(iii). Other applications of bioluminescence imaging
[edit | edit source]Bioluminescence imaging has been applied in experimental biomedical research, e.g., development of necrosis, and in other areas of biology.[20] It has also been used particularly on tumour biopsies in clinical oncology. In combination with immunohistochemistry,autoradiography or in situ hybridization it can be particularly powerful. It has been shown for squamous cell carcinomas that accumulation of lactate in the primary lesions is associated with a high risk of metastasis. In this way, metabolic mapping indicates the degree of malignancy and the prognosis of tumours; it has stimulated a number of fundamental investigations.
e(iv). Other methods of determination of metabolites
[edit | edit source]There are numerous other examples of methods to determine metabolites in living cells and tissues, including real-time imaging of metabolite production. Endogenous acetylcholinesterase (ACE) activity has been detected in rat coronal brain slices using coupled reactions with choline oxidase and horseradish peroxidase.[21] The reagent is optimized to minimize diffusion of emitting species, giving sharp localization and a very low background. This imaging assay is more predictive than in vitro systems; and can be used to determine pathophysiological changes in ACE distribution or the effect of in vivo ACE inhibitors, which could be useful for screening candidate drugs.
Nitric oxide (NO) released from cell cultures and living tissue has been visualized by a reaction with luminol and hydrogen peroxide to yield photons which were counted using a microscope coupled to a photon counting camera, giving new insight into release time course and diffusion profile.[22] The method allowed integration times in the order of minutes to improve signal-to-noise ratio. However, the high sensitivity of this method also makes it possible to generate an image in seconds, allowing the production of real time moving pictures. This method has demonstrated potential for real time imaging of NO formation, with high temporal and spatial resolution. There was little earlier knowledge of this phenomenon due to the short half-life of NO.
D6f. In situ hybridization (ISH) and immunohistochemistry (IHC)
[edit | edit source]ISH and IHC are techniques that localize analytes in a wide range of suitable specimens such as cellular smears, or frozen or paraffin-embedded sections. Chemiluminescence detection does not require any special specimen preparation, but an accurately controlled section thickness of from 3 to 5 μm is necessary for reproducibility. Incorporating chemiluminescence detection (CL-IHC and CL-ISH) increases sensitivity compared with colorimetry or fluorometry. This adds reliable and accurate quantitative evaluation of spatial distribution to the specificity of the probe. The “theoretical” limit of detection of the enzyme label by chemiluminescence is 10−21 to 10−18 mol; as a detector for ISH,chemiluminescence is almost as sensitive as 35S autoradiography giving a nontoxic alternative to the use of radioactivity.[23]
Localization within cells of nucleic acid sequences, e.g., the sites of genes in chromosomes, can be achieved by hybridization to complementary nucleic acid probes. The two general types of in situ hybridization involve nuclear DNA and cellular RNA respectively; they are conceptually similar but differ in practical detail. The technique is usually performed on specimens prepared for light microscopy. It is claimed that little or no microscopic training is necessary to evaluate the chemiluminescence images.

Fig. D6.1 – Flow chart of operations for the performance of in situ hybridization. (All incubations are at room temperature).
f(i). CL-ISH assay of human papilloma virus (HPV)
[edit | edit source]ISH involves nucleic acid probe hybridization with DNA or RNA, endogenous to the specimen or exogenous (viral/bacterial). The procedure is summarized in Fig. D6.1. Sensitivity is increased by indirect labelling, in which the label binds with a biospecific chemiluminescence reagent, e.g., biotin binds to streptavidin; fluorescein or digoxigenin bind to their respective antibodies, the chemiluminescence reagent having a covalently-bound signalling group (usually AP or HRP). ISH of HPV can be imaged using a digoxigenin-labelled gene probe followed by an HRP-labelled anti-digoxigenin antibody and a chemiluminescence reagent.[24] To localize the virus, the chemiluminescence image (with intensities represented by pseudocolours) is overlayed onto a transmitted light image. ISH has also been performed on three human carcinoma cell lines and 40 biopsy specimens of human cervical neoplastic and preneoplastic lesions by using biotin-labelled complementary DNA probes of HPV, detected by HRP-labelled secondary antibodies; the chemiluminescence was detected by an ICCD camera.[15] After only 10 min of photon accumulation, on cell line smears as well as on serial tissue sections, chemiluminescence gave comparable results to those obtained by a 3-week exposure for 35S-autoradiography.
CL-ISH is quantitative because chemiluminescence is proportional to the enzyme activity of the label, and to the number of gene copies per cell. In a separate study of cytomegalovirus, chemiluminescence was proportional to the number of cells infected (following virus replication).
f(ii). CL-ISH of cytomegalovirus
[edit | edit source]An early ISH assay of cytomegalovirus DNA in infected human fibroblasts[13] used dioxigenin-labelled probes and AP-labelled anti-digoxigenin antibody. Employing a low-light imaging luminograph 400 amol of AP were detected using 1,2-dioxetanes. Chemiluminescence was intense and stable, making possible quantitation within single cells, with a spatial resolution of 1 μm and very low background. Multiplexed CL-ISH assays have been developed in which probes with different enzyme labels detect different targets. One example of such techniques localizes the DNA of herpes simplex and cytomegalovirus in the same specimen using the following protocol. The faster HRP/luminol is added to the specimen and chemiluminescence is imaged, the specimen is given a short wash, then AP/dioxetane is added, and a second chemiluminescence image is recorded. A longer wash is needed if AP is added first.
f(iii). CL-ISH of parvovirus B19 nucleic acids in single infected cells
[edit | edit source]Human parvovirus B19 is responsible for a wide range of diseases. CL-ISH gives high resolution, providing precise localization and quantitative detection of the viral nucleic acids in single cells in cultures at different times after infection, giving an objective evaluation of the infection process with higher sensitivity than colorimetric ISH detection assessed by panels of observers. The improved sensitivity of CL-ISH detects more positive cells per sample, making possible earlier diagnosis.
A peptide nucleic acid (PNA) has been developed which has improved specificity and faster, stronger binding than other DNA probes. The assay is based on the use of a biotin-labelled PNA probe which is detected by a streptavidin-linked alkaline phosphatase (AP), using the well-known biotin-streptavidin affinity:
PNA–biotin + streptavidin–AP → PNA–biotin–streptavidin–AP
adamantyl 1,2-dioxetane phosphate + AP → excited fragmentation products → light
The chemiluminescence signal which arises was quantified and imaged with an ultrasensitive nitrogen-cooled CCD camera connected to an epifluorescence microscope with high-transmission optics and modified for acquisition of chemiluminescence. A threshold signal (representing non-specific binding of the probe and endogenous alkaline phosphatase activity) was established using mock-infected cells as negative controls. Following a B19 virus infectious cycle the percentage of infected cells, which reached its maximum at 24 h after infection, could be accurately monitored. The advantages of chemiluminescence detection (high detectability and wide linear range) allow the quantitative analysis of viral nucleic acids in infected single cells, showing a continuous increase with time after infection. Such investigations could be powerful tools for the assessment and diagnosis of viral infections and for measuring the virus load of infected cells.[25]
f(iv). IHC with chemiluminescence detection (CL-IHC)
[edit | edit source]IHC involves the use of antibodies that bind to endogenous, viral or bacterial antigens (a protein usually) with subsequent detection by enzyme-conjugated antibodies. CL-IHC detects epithelium in thyroid tissue by HRP-labelled antibodies and luminol/H2O2, with adequate resolution and greater sensitivity than colorimetry or fluorescence. CL-IHC can also with advantage be applied to Interleukin 8 (IL-8) localization in gastric biopsy specimens infected by Helicobacter pylori, an organism associated with gastric ulcers. It shows with greater sensitivity than other detection systems the variability of the IL-8 concentration in the mucosa and the foci of high concentration in the epithelial cells.

Fig. D6.2 – Flow chart of operations for the performance of immunohistochemistry. (All incubations are at room temperature).
f(v). HPV and p16(INK4A) marker in cervical cancer
[edit | edit source]Cervical cancers (cervical intraepithelial neoplasms, CIN) are classified into low- (CIN1) or high-grade (CIN2 or CIN3) in order to predict the risk of progression of early lesions and enable decisions to be made concerning surgical intervention. Judgments based on histology are imprecise in that different observers assign different grades to the same biopsy specimen. One way of overcoming this difficulty is to redefine the diagnostic criteria in terms of analytical chemistry.
Figure D6.3 - Diagrammatic representation of the localization of p16INK4A by CL-IHC in a biopsy section of a cervical cancer. Paler shades show increased chemiluminescence emission. (An actual chemiluminescence image is shown in Fig. 1 in reference 27, on which this diagram is based.)
An immunohistochemical assay (see Fig. D6.2) with chemiluminescence detection (CL-IHC) has been used to quantitatively evaluate the overexpression of the protein p16INK4A and its localization in the epithelium of samples from cervical cancers and from non-cancerous cervical lesions. Fig. D6.3 shows that chemiluminescence (and hence p16INK4A protein content) generally increases from left to right. High-grade lesions give generally more intense chemiluminescence signals in the epithelium than low-grade and show a different distribution of p16INK4A protein. From the intensity of the chemiluminescence signal and the percentage of the epithelium involved in the overexpression of p16INK4A an expression score was obtained which discriminated well among different lesions. A cut-off value was determined to distinguish between low and high grades. The differences between the average scores of different CIN grades were statistically significant.[26]

Figure D6.4 - Diagrammatic representation of the co-localization of p16INK4A and HPV DNA in a tissue section from a cervical biopsy. (A) transmitted light microphotographic image, (B) CL-IHC image, the lighter tones showing p16INK4A, (C) CL-ISH image, the lighter tones showing HPV DNA, (D) CL-ISH image pseudocoloured in blue, yellow and red to indicate increasing chemiluminescence intensity. (B), (C) and (D) show images overlaid onto a transmitted light image to show the localization of the signal. (Actual chemiluminescence images are shown in Figs. 3 and 4 in reference 27, on which this diagram is based.)
The determination of p16INK4A overexpression by CL-IHC used AP as the enzyme label and then, after washing in buffer, HPV DNA was determined by CL-ISH[24] (see Fig. D6.1 and section f(i) ADD LINKS) using HRP as label to avoid interference between the two assays.[27] To circumvent the non-equivalence of consecutive tissue sections, the two assays were carried out on the same sample. The assays cannot be carried out in the reverse order as the high-temperature step in ISH denatures the p16 protein to be determined by IHC. The high detectability of chemiluminescence gives improved discrimination between lesions as non-cancerous, CIN1 or high-grade CIN. This could become an objective and accurate diagnostic test.
f(vi). Mucosal human papilloma virus in malignant melanomas
[edit | edit source]High-risk (HR) mucosal human papilloma virus (HPV) is strongly associated with cancer. It has been found in primary melanoma and in pigmented skin blemishes (birthmarks, moles) but has rarely been reported in normal skin, which is instead commonly infected with other relatively harmless strains of HPV. HPV DNA in skin cancer has been detected by polymerase chain reaction (PCR). In order to understand the relationship between HPVs and primary melanoma, it is necessary to know whether the presence of HPV is localized in cancer cells rather than in normal skin cells present in the tumour biopsy, what proportion of the cells harbours the virus or whether it can be due to contamination of the tumour surface by viruses from healthy skin. Because PCR methods measure only total DNA they are not suitable to ascertain this.

Fig. D6.5 – Schematic representation of a tissue section that has undergone the combined procedures of FL-ISH for HPV DNA and CL-IHC for tumour marker HMB-45. The large coloured dots represent cells. Red pseudocolour was assigned to chemiluminescence signals and yellow to fluorescence signals, different signal intensities represented by different shades. Colocalization of HPV and HMB-45 is represented by the combined pseudocolour, orange.
To localize HR-HPV a rapid, specific and very sensitive method has been developed that combines an enzyme-amplified fluorescence in situ hybridization (FL-ISH, see Fig. D6.1 ADD LINK) for the detection of HPV nucleic acids (types 16 and 18, which are the types most likely to cause cancer) with a chemiluminescence immunohistochemistry (CL-IHC, see Fig. D6.2 ADD LINK) method for the detection sequentially in the same section of the tumoural melanocytic marker HMB-45. It is necessary to use the same section because the melanoma cells are distributed heterogeneously in the specimens. HMB-45 determination is an indicator of melanoma cell differentiation and is widely used in diagnostic pathology. Digital images of FL-ISH and CL-IHC were separately recorded, assigned different pseudocolours (see Fig. D6.5) and merged using specific software for image analysis. The results demonstrated a sharp colocalization (to an extent of about 70% of the total luminescent area of the specimen) of HPV nucleic acids and the melanoma marker in the same biopsy sections. In smaller areas, HPV was detected without HMB-45 (9.5% of total) or HMB-45 without HPV (20.5%). This demonstrates that viral nucleic acids were specifically present in melanoma cells and supports a possible active role of HPV in malignant melanoma.[28]
D6g. Chemiluminescence imaging of fluorescence reporters
[edit | edit source]Fluorescence detection remains of great value for in situ hybridization and in immunohistochemistry, particularly because of its greater precision of spatial localization compared with chemiluminescence. Chemiluminescence can, however, be harnessed as a means of exciting fluorescent probes and labels alternative to photo-excitation. Two examples of the principle are considered in this section.
g(i). Peroxy-oxalate chemiluminescence
[edit | edit source]Using imaging chip-based devices, detection of aqueous peroxyoxalate chemiluminescence (POCL) from oxamide I in aqueous environments has been reported[29] for fluorescence-labelled analytes and proved to be at least as sensitive as that using direct fluorescence detection requiring a light source for excitation. Using a CCD camera to record the chemiluminescence intensity from a 1000-fold range of analyte concentrations, POCL detection sensitivity of fluorescence-labelled immunoglobulins on a nitrocellulose membrane was investigated. Aqueous POCL of Staphylococcus aureus enterotoxin B (SEB) and its antibody were also used to demonstrate immuno- and affinity-detection using a CCD camera. SEB was detected by an immune sandwich assay in which SEB was captured by sheep polyclonal antibody spotted onto a nitrocellulose membrane and subsequently captured mouse monoclonal antibody, which was detected by fluorescence-labelled anti-mouse antibody. Affinity detection of biotin-labelled anti-SEB antibody used fluorescence-labelled streptavidin.
Simultaneous detection by POCL of bovine serum albumin labelled with two different fluorescent labels has been demonstrated, using contact imaging with a CMOS colour imaging chip (ADD LINK). The proteins were spotted onto a membrane disc fixed to a cover slip which was placed on the sensing surface of the chip. They were visible on 8 s exposure as red and green spots respectively; a mixture of the labelled samples emitted yellow light. This procedure might be applicable to reading microarrays.
g(ii). Bioluminescence resonance energy transfer (BRET)
[edit | edit source]A self-illuminating fluorescence reporter, comprising a dye conjugated to AP, has been demonstrated “in principle” for imaging detection using a CCD camera or a CMOS colour chip, making possible the imaging of fluorescent signals without the need for an external light source or sophisticated optics.[30] It is based on bioluminescence resonance energy transfer (BRET), an example of which, already cited, is the use of GFP to enhance the light emission from the photoprotein aequorin (see section d(iii) ADD LINK). The efficiency of BRET is increased by minimizing the distance between the bioluminescent energy donor and the fluorescent acceptor and is also found to depend on the ratio of AP to fluorophore in the conjugate, on the fluorescent dye used and on the chemiluminescent substrate. Chemiluminescence detection is low-cost, suitable for low concentrations and portable but diffusion of the luminescent products leads to poor spatial resolution. BRET is a potential solution to this problem, but it has not yet been applied to a real analytical problem.
In the demonstration, antibody, immobilized on the CMOS surface, captured a biotin-labelled target molecule that was then bound to the streptavidin-labelled AP-dye conjugate. The AP was used to generate light and the captured array images were viewed on a computer monitor. Images were also obtained by using a CCD camera. The chemiluminescent substrate for AP emitted at 450 nm; the energy from this emission was transferred to the fluorescent dye. This resulted in a second light emission with a longer wavelength (580 nm), which was localized at the position of target molecules, avoiding the problem of diffusion of the chemiluminescent product. In this way, image spatial resolution was greatly improved compared with conventional chemiluminescence detection. The shorter wavelength first emission that escaped absorption by the dye was removed by a high pass filter.
D6h. Whole-organ and whole-organism imaging
[edit | edit source]The use of cameras remote from the site of light emission makes it possible to image events occurring in the interior of organs and organisms. This technique can be applied to the study of a wide range of phenomena such as tumour growth, metastasis and drug efficacy, assessed by injecting and imaging recombinant light-emitting tumour cells,[31] which can be used as probes for tumour location. Another application of molecular imaging techniques is the non-invasive monitoring of transplanted embryonic cardiomyoblasts expressing firefly luciferase (Fluc) reporter gene.[32] The movement in the rat gut of bioluminesent E. coli (expressing luciferase and the enzyme necessary for substrate synthesis) has also been imaged.[33]

Fig. D6.6 – Diagrammatic representation of Renilla luciferase expression in a transgenic tobacco leaf, imaged against a dark background.. Light emission is indicated by white or grey areas. (The diagram is based on the image reproduced in figure 1 of reference 34)
Luciferase enzymes to label cells, pathogens, and genes are internal indicators that can be detected externally. Transgenic organisms have been produced in which the gene for the luciferase of Renilla reniformis functions stably in tobacco (Nicotiana tabaca), tomato (Lycopersicon exculentum) and potato (solanum tuberosum). Strong light emission was imaged with a low-light video camera after only a few seconds immersion of leaves, slices and seedlings (see Fig. D6.6) in 3 μmol dm−3 2-benzyl luciferin solution; at this concentration, the substrate was nontoxic and no other abnormalities were apparent.[34]
Luciferase imaging enables complex gene activation effects to be modelled and observed in live animals.[19] Bioluminescent reporters for given biological processes have been used widely in cell biology; in whole animal models, including light-producing transgenic animals as models of disease, they are useful in drug discovery and development. In vivo imaging of intact organs also furthers the understanding of biological processes. The application of this technology to living animal models of infectious disease has provided insights into disease processes, therapeutic efficacy and new mechanisms by which pathogens may avoid host defences.[35] Progress of infections and efficacy of treatment are assessed by bioluminescent markers, e.g., light-emitting pathogens. Rapid, accessible high throughput screening is effective in vivo for pharmacokinetics, toxicology and target validation. Study of spatio-temporal patterns helps to characterize the site and time of action of drugs. There is also a possible clinical use in gene therapy and assessing gene vaccine delivery and efficacy.
There are several advantages in the use of in vivo imaging. In continuous monitoring each animal serves as its own control – introducing less variability than comparing groups of animals each analyzed at a different time; in addition, fewer animals are used in the experiments. Multiplex in vivo assays are also possible, using two or more reporters in the same animal. Chemiluminescence imaging is validated by the correlation of bioluminescence with culture cell counts (e.g., of E. coli). Investigations of this kind increase the number of data and can guide tissue sampling for subsequent biochemistry or histology.
There are also several drawbacks. Red and infrared light (590-800 nm) penetrates tissue well, but blue/green light (400-590 nm), usually the bulk of the bioluminescence emission, is strongly attenuated. Luciferase, however, is the most widely-used reporter and has a broad emission including red light. The spatial resolution (3–5 mm) is worse than in magnetic resonance imaging or computed tomography.
Some pathological processes result spontaneously in light production. This is due to a weak spontaneous photon emission associated with oxidative phenomena, such as oxygen free radical (OFR) formation (ADD LINK), in whole organs removed from living animals. OFR have been imaged in rat livers that have been subjected to ischaemia (occlusion of blood supply) and reperfusion (restoration of blood supply), showing distribution in space and time of superoxide radicals on the liver surface and the effects on them of antioxidants, which remove OFR and can be screened by this model. The roles of aging, ethanol consumption and fat deposition on OFR formation in the liver have also been assessed. This system can be used to monitor the storage of organs for transplantation and to test agents and procedures for preserving them.
References
[edit | edit source]- ↑ a b Roda A, Guardigli M, Pasini P, Mirasoli M, Michelini E, Musiani M, Bio- and chemiluminescence imaging in analytical chemistry, Anal. Chim. Acta, 2005, 541, 25-36.
- ↑ Roda A, Pasini P, Baraldini M, Musiani M, Gentilomi G, Robert C, Chemiluminescent imaging of enzyme-labelled probes using an optical microscope-videocamera luminograph, Anal. Biochem. 1998, 257(1), 53-62.
- ↑ a b Vykoukal D M, Stone G P, Gascoyne P R C, Alt E U and Vykoukal J, Quantitative detection of bioassays with a low-cost image-sensor array for integrated microsystems, Angew. Chem. Int. Ed., 2009, 48, 7649-7654.
- ↑ Creton R, Kreiling J A, Jaffe L F, Calcium imaging with chemiluminescence, Microscopy Research and Technique, 1999, 46(6), 390-397.
- ↑ S. Morel and L. Koechlin, The DELTA photon counting camera concept, Astron. Astrophys. Suppl. Ser., 1998, 130, 395-401.
- ↑ Roda A, Mirasoli M, Venturoli S, Cricca M, Bonvicini F, Baraldini M, Pasini P, Zerbini M, Musiani M, Microtiter format for simultaneous multianalyte detection and development of a PCR-chemiluminescent enzyme immunoassay for typing human papillomavirus DNAs, Clin. Chem., 2002, 48(10), 1654-1660.
- ↑ Sun Z H, Fu X L, Zang L, Yang X L, Liu F Z, Hu G X, A protein chip system for parallel analysis of multi-tumor markers and its application in cancer detection, Anticancer Research, 2004, 24(2C), 1159-1165.
- ↑ Feissner R, Xiang Y B, Kranz R G, Chemiluminescent-based methods to detect subpicomole levels of c-type cytochromes, Anal. Biochem., 2003, 315(1), 90-94.
- ↑ Huang G M, Ouyang J, Delanghe J R, Baeyens W R G, Dai Z X, Chemiluminescent image detection of haptoglobin phenotyping after polyacrylamide gel electrophoresis, Anal. Chem., 2004, 76(11), 2997-3004.
- ↑ Wang L, Zhang Z J, Huang L G, Molecularly imprinted polymer based on chemiluminescence imaging for the chiral recognition of dansyl-phenylalanine, Anal. Bioanal. Chem., 2008, 390(5), 1431-1436.
- ↑ Holthoff E L and Bright F V, Molecularly templated materials in chemical sensing, Anal. Chim. Acta, 2007, 594, 147-61.
- ↑ Wang L, Zhang Z J, Chemiluminescence imaging assay dipyridamole based on molecular imprinted polymer as recognition material, Sensors and Actuators B-Chemical, 2008, 133(1), 40-45.
- ↑ a b Roda A, Pasini P, Musiani M, Girotti S, Baraldini M, Carrea G, Suozzi A, Chemiluminescent low-light imaging of biospecific reactions on macro- and microsamples using a videocamera-based luminograph, Anal. Chem., 1996, 68(7), 1073-1080.
- ↑ Creton R, Jaffe L F, Chemiluminescence microscopy as a tool in biomedical research, Biotechniques, 2001, 31(5), 1098.
- ↑ a b Baubet V, Le Mouellit H, Campbell A K, Lucas-Meunier E, Fossier P, Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level, Proc. Natl. Acad. Sci., 2000, 97(13), 7260-7265.
- ↑ Solovyova N, Verkhratsky A, Monitoring of free calcium in the neuronal endoplasmic reticulum: an overview of modern approaches, J. Neurosci. Meth., 2002, 122(1), 1-12.
- ↑ Mueller-Klieser W, Walenta S, Geographical mapping of metabolites in biological tissue with quantitative bioluminescence and single-photon imaging, Histochem. J., 1993, 25(6), 407-420.
- ↑ Levin M, Leppanen O, Evaldsson M, Wiklund O, Bondjers G, Bjornheden T , Mapping of ATP, glucose, glycogen, and lactate concentrations within the arterial wall, Arteriosclerosis, Thrombosis and Vascular Biology, 2003, 23(10), 1801-1807.
- ↑ a b Greer LF, Szalay AA, Expression of luciferases in living cells and organisms, Luminescence, 2002, 17(1), 43-74.
- ↑ Walenta S, Schroeder T, Mueller-Klieser W, Metabolic mapping with bioluminescence: basic and clinical relevance, Biomolecular engineering, 2002, 18(6), 249-262.
- ↑ Pasini P, Musiani M, Ruso P, Valenti P, Aicardi G, Crabtree J E, Baraldini M, Roda A, Chemiluminescence imaging in bioanalysis, J. Pharm. Biomed. Anal., 1998, 18, 555.
- ↑ Wiklund N P, Iversen H H, Leone A M, Cellek S, Brundin L, Gustafsson L E, Moncada S, Visualization of nitric oxide formation in cell cultures and living tissue, Acta Physiologica Scandinavica, 1999, 167(2), 161-166.
- ↑ Lorimier P, Lamarcq L, Negoescu A, Robert C, LabatMoleur F, GrasChappuis F, Durrant I, Brambilla E, Comparison of S-35 and chemiluminescence for HPV in situ hybridization in carcinoma cell lines and on human cervical intraepithelial neoplasia, Journal of Histochemisry and Cytochemistry, 1996, 44(7), 665-671.
- ↑ a b See Figs. 3 and 4 in reference 1.
- ↑ Bonvicini F, Mirasoli M , Gallinella G, Zerbini M, Musiani M, Roda A, PNA-based probe for quantitative chemiluminescent in situ hybridization imaging of cellular parvovirus B19 replication kinetics, Analyst, 2007, 132(6), 519-523.
- ↑ Venturoli S, Ambretti S, Mirasoli M, Santini D, Zerbini M, Roda A, Musiani M , Chemiluminescent quantitative immunohistochemical p16INK4A localization as a marker for cervical intraepithelial neoplasias, International Journal of Gynecological Pathology, 2008, 27(4), 575-581.
- ↑ Mirasoli M, Guardigli M, Simoni P, Venturoli S, Ambretti S, Musiani M, Roda A, Multiplex chemiluminescence microscope imaging of p16INK4A and HPV DNA as biomarker of cervical neoplasia, Anal. Bioanal. Chem., 2009, 394, 981-987.
- ↑ Ambretti S, Venturoli S, Mirasoli M, La Placa M, Bonvicini F, Cricca M, Zerbini M, Roda A, Musiani M, Assessment of the presence of mucosal human papillomaviruses in malignant melanomas using combined fluorescent in situ hybridization and chemiluminescent immunohistochemistry, British Journal of Dermatology, 2007, 156(1), 38-44.
- ↑ Filanoski B, Rastogi SK, Cameron A, Cameron E, Mishra NN, Maki W, Maki G, Non-enzymatic aqueous peroxyoxalate chemiluminescence immune detection using a CCD camera and a CMOS device, Luminescence, 2008, 23(5), 296-302.
- ↑ Filanoski B, Rastogi SK, Cameron E, Mishra NN, Maki W, Maki G, A novel homogeneous bioluminescence resonance energy transfer element for biomolecular detection with CCD camera or CMOS device, Luminescence, 2008, 23(1), 22-27.
- ↑ Soling A, Rainov NG, Bioluminescence imaging in vivo - application to cancer research, Expert Opin. Biol. Ther., 2003, 3, 1163.
- ↑ Wu J C, Chen I Y, Sundaresan G, Min J J, De A, Qiao J H, Fishbein M C, Gambhir S S, Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography, Circulation, 2003, 108, 1302.
- ↑ See figure 5 in reference 1.
- ↑ Mayerhofer R, Wang G, Hua D, Escher A, Illes K, Langridge W H R, Szalay A A, Visualization of light emission from different luciferases to transgenic organisms, in Campbell A K, Kricka L J, Stanley P E (eds.), Bioluminescence and Chemiluminescence: Fundamentals and Applied Aspects. Proceedings of the 8th international symposium on bioluminescence and chemeiluminescence 1994 [Chichester, Wiley. 1995], 607-612.
- ↑ Doyle TC, Burns SM, Contag CH, In vivo bioluminescence imaging for integrated studies of infection, Cellular Microbiology, 2004, 6(4), 303-317.
Electrochemiluminescence
D7. Electrochemiluminescence
[edit | edit source]Electrochemiluminescence is chemiluminescence arising as a result of electrochemical reactions. It includes electrochemical initiation of ordinary chemiluminescent reactions, electrochemical modification of an analyte enabling it to take part in a chemiluminescent reaction, or electron transfer reactions between radicals or ions generated at electrodes. Prominent in the work done on electrochemiluminescence are reactions involving polyaromatic hydrocarbons or transition metal complexes, especially those of ruthenium, palladium, osmium and platinum.
Applications have made use of the sensitivity, selectivity and wide working range of analytical chemiluminescence, but electrochemiluminescence offers additional advantages without adding much to the inexpensive instrumentation.[1] Electrodes can be designed to achieve maximum detection of the light emitted and electrochemical measurements can be made simultaneously with the light output. Generation of chemiluminescence reagents at electrodes gives control over the course of light producing reactions, which can effectively be switched on and off by alteration of the applied potential; this is particularly useful when using unstable reagents or intermediates. Other possible benefits include generation of reagents from inactive precursors and regeneration of reagents, which permits the use of lower concentrations or immobilization of the reagents on the electrode. Analytes can also be regenerated, so that each analyte molecule can produce many photons, increasing sensitivity, or they can be modified to make them detectable by the chemiluminescence reaction in use. Electrochemiluminescence can be coupled with high performance liquid chromatography or with capillary electrophoresis.
The usefulness of tris-(2, 2/-bipyridyl)ruthenium(II) (discussed in chapter B9 ADD LINK) in electrochemiluminescence rests on its activity with very high efficiency at easily accessible potentials and ambient temperature in aqueous buffer solutions in the presence of dissolved oxygen and other impurities. The reaction sequence that leads to electrochemiluminescence is shown in equations D7.1 to D7.4:
(D7.1) Oxidation: [Ru(bipy)3]2+ ─ e― → [Ru(bipy)3]3+
(D7.2) Reduction by analyte: [Ru(bipy)3]2+ + e― → [Ru(bipy)3]+
(D7.3) Electron transfer: [Ru(bipy)3]3+ + [Ru(bipy)3]+ → [Ru(bipy)3]2+ + [Ru(bipy)3]2+*
(D7.4) Chemiluminescence: [R(bipy)3]2+* → [Ru(bipy)3]2+ + light

Figure D7.1 – A flow injection manifold for measuring electrochemiluminescence.
The oxidation occurs electrochemically at the anode, whereas the reduction is brought about chemically by the analyte in the free solution. Electron transfer and subsequent chemiluminescence also occur in the free solution close to the anode, where the [Ru(bipy)3]3+ is concentrated. Other analytes, e.g. alkylamines, are oxidized at the anode to form a highly reducing radical intermediate that reacts with [Ru(bipy)3]3+ to form [Ru(bipy)3]2+*, which emits light. Oxalates, on the other hand, are oxidized by [Ru(bipy)3]3+ to radicals that then reduce more [Ru(bipy)3]3+ to give [Ru(bipy)3]2+* and chemiluminescence.
Instrumentation for electrochemiluminescence differs from that for other chemiluminescence only in having a flow cell provided with working, counter and reference electrodes, regulated by a potentiostat, which is in turn controlled by the computer that receives input from the photomultiplier or other transducer that receives the light signals. Figure D7.1 shows the usual flow injection manifold used for measuring electrochemiluminescence. The flow cell is in a light-tight box to exclude ambient light. A more portable alternative is a probe containing a set of electrodes and a fibre optic bundle to carry emitted light to a photomultiplier. Ambient light is excluded by means of baffles in the channels that admit the test solution. Because it can be electrochemically regenerated, it is useful to immobilize [Ru(bipy)3]3+ in a cation exchange resin deposited on the electrode to form a sensor that does not need a continual reagent supply.
References
[edit | edit source]- ↑ Knight A W, A review of recent trends in analytical applications of electrogenerated chemiluminescence, Trends Anal. Chem., 18(1), 1999, 47-62.
Photo-induced chemiluminescence
D8. Photo-induced chemiluminescence
[edit | edit source]Photo-induced chemiluminescence (PICL) involves irradiating an analyte with ultra-violet light in order to convert it to a photoproduct of different chemiluminescence behaviour, usually substantially increased emission. Such reactions form the basis of highly sensitive and selective analytical techniques. Irradiating a molecule can break it into fragments of smaller molecular weight (photolysis) or can induce reactions such as oxidation, reduction, cyclization or isomerization. Direct photolysis involves absorption of photons by the target molecule; in indirect photolysis, the target molecule absorbs energy from another molecule that has previously absorbed photons.[1]
Photochemistry is concerned with excited electronic states induced by the absorption of photons. Photoexcitation is more selective than thermal excitation and leads to a different energy distribution within the molecule. The excited molecule can undergo photochemical processes, the products of which are sometimes involved in side processes. In analytically useful photochemical reactions the light is strongly absorbed by the analyte but not by the photoproducts; the photochemical yield is high; the photoproducts are stable for as long as is needed to complete the analysis and are structurally rigid enough for the emission to have an adequate quantum yield. Successful analytical application also depends on appropriately designed photoreactors. When those conditions are fulfilled, using light has several advantages over the use of chemical derivatization. Lamps are inexpensive and their stable light output allows reproducible results. They differ in their spectral characteristics, which gives scope for increasing selectivity. The use of light has minimal environmental impact and can be effected in ambient conditions. Analysis times are shorter because photochemical reactions are fast and can be shortened further by optimizing reactor configuration or increasing lamp power. PICL has a linear relationship with analyte concentration over a wide concentration range and extends the range of analytes that can be detected by chemiluminescence. It is not necessary to identify or separate the photoproducts.
In PICL-based methods, the sample is irradiated on-line and subsequently merged with the chemiluminescence reagents prior to reaching the flow cell in front of the detector. Flow methods allow the irradiation time to be easily controlled and provide better reproducibility than stationary methods, coping better with the very fast rate of chemiluminescence reactions. Sample throughput, ease of automation and reagent consumption are also improved using flow methods.
PICL has the same instrumentation as other chemiluminescence but, in addition, a photoreactor is required and this has two essential elements – a light source and a container for the sample. Lamps are selected on the basis of power and spectrum (continuous or discrete). Continuous spectra span a wide zone, whereas discrete spectra are series of individual lines in a narrow wavelength range. The mercury-xenon lamp provides a continuous spectrum and is used when its high power is necessary, though it needs cooling. The low-pressure mercury lamp generates little heat and is a typical discrete-spectrum lamp, emitting over the range 200-320 nm, maximally at 254 nm; most substances absorb in this zone. The absorption zone of the selected lamp must be the most useful for excitation and bond-breaking. In flow systems, the sample is usually contained in PTFE tubing, which admits little light but maximises its effect by repeated reflections from the inner tube surfaces; the tubing can be coiled around a low-power lamp. Batch methods instead use quartz cells, which are transparent to ultra-violet. Quartz is more inert than PTFE, but also more fragile.
References
[edit | edit source]- ↑ Icardo M C and Catalyud J M, Crit. Rev. Anal. Chem., 38, 2008, 118-130.
Chemiluminescence detection in gas chromatography
D9. Chemiluminescence detection in gas chromatography
[edit | edit source]
Figure D9.1 – Flow chart of a gas chromatograph.
Gas chromatography is a major separation method. It consists of the injection of a gaseous or liquid sample into a gaseous mobile phase which is passed through a column of solid support particles carrying a liquid stationary phase, maintained in an oven at a suitable temperature (which is usually above ambient but need not be above the boiling points of the analytes). Separation is the result of partition between the stationary and mobile phases and the separated constituents of the sample are usually detected by a flame ionization detector. A flow chart of the typical instrumentation for gas chromatography is illustrated in figure D9.1.
Samples of increasing complexity are being analysed by gas chromatography. Universal detectors, such as the flame ionization detector, are not adequate for such a task but selective detectors can provide the additional discrimination that is needed. Nitrogen- and sulfur-containing compounds commonly occur as trace-level analytes in complex samples and highly selective detectors have been developed. Among these, the nitrogen chemiluminescence detector and the sulfur chemiluminescence detector have emerged as powerful tools in gas chromatography, supercritical fluid chromatography and high performance liquid chromatography; stand-alone nitrogen/sulfur analysers can be based on the same chemiluminescence reactions. Detectors of either element are based on the same ozone-induced gas phase chemiluminescence.[1] The chemiluminescence is preceded by high temperature pyrolysis which oxidizes the nitrogen in the sample (RN) to nitric oxide (NO):
(D9.1) Oxidation: RN + O2 → NO + CO2 + H2O
and it is believed that the sulfur in the sample (RS) is converted first into sulfur dioxide (SO2), which is then reduced in the presence of hydrogen to sulfur monoxide (SO):
(D9.2) Oxidation: RS + O2 → SO2 + CO2 + H2O
(D9.3) Reduction: SO2 + H2 → SO + H2O
(D9.4) Overall: RS + O2 + H2 → SO + CO2 + H2O
These reactions produce the species that react with ozone, producing excited nitrogen dioxide and excited sulfur dioxide respectively (eqns. D9.5 and D9.7):
(D9.5) Reaction with ozone: NO + O3 → NO2* + O2
(D9.6) Chemiluminescence: NO2* → NO2 + light (~ 1200 nm)
(D9.7) Reaction with ozone: SO + O3 → SO2* + O2
(D9.8) Chemiluminescence: SO2* → SO2 + light (~ 360 nm)
The nitrogen chemiluminescence reaction emits in the near infra-red (eqn. D9.6), whereas the sulfur reaction emits in the ultra-violet (eqn. D9.8). This wide spectral separation of the emission bands enables nitrogen and sulfur to be determined selectively. A few small gaseous molecules containing sulfur also enter into the chemiluminescent reaction with ozone without undergoing preliminary pyrolysis.

Figure D9.2 – Flow diagram of a nitrogen-sulfur detector.
The instrumentation for nitrogen-sulfur chemiluminescence detection is depicted in figure D9.2. The pyrolyser converts the analytes in the gas chromatograph column effluent into the corresponding chemiluminescent species, which pass to the reaction chamber where they react with ozone supplied by a generator. The light emitted is detected by a photomultiplier.
References
[edit | edit source]- ↑ Yan X, J. Sep. Sci., 2006, 29, 1931.
Chemiluminescence detection in high performance liquid chromatography
D10. Chemiluminescence detection in high performance liquid chromatography
[edit | edit source]
Figure D10.1 – Flow chart of high performance liquid chromatography with chemiluminescence detection.
High performance liquid chromatography consists of the injection of a liquid sample into a liquid mobile phase which is passed through a column of solid or supported liquid stationary phase. Separation is the result of partition, adsorption, size exclusion or ion exchange between the stationary and mobile phases and the separated constituents of the sample are usually detected by an ultra-violet absorption detector. A flow chart of the typical instrumentation used is illustrated in figure D10.1.
Coupling with chemiluminescence detection adds the sensitivity of this technique to selectivity of a powerful separation method. It requires measurement of the emitted light due to a post-column reaction between the analytes in the column eluents and the chemiluminescence reagents, which are delivered by additional pumps with the incorporation of devices for rapid mixing. Measurement of the chemiluminescence intensity at its maximum requires optimization of the transit time (dependent on length of tubing and flow rate) between the mixing point and the detector. The most important problem in designing the coupling instrumentation is ensuring compatibility between the conditions necessary for efficient chromatographic separation and those needed for intense chemiluminescence. Separation depends heavily on mobile phase composition, whereas chemiluminescence emission is known to be affected by solvent, pH, reaction temperature and the presence of enhancers and/or catalysts.[1]
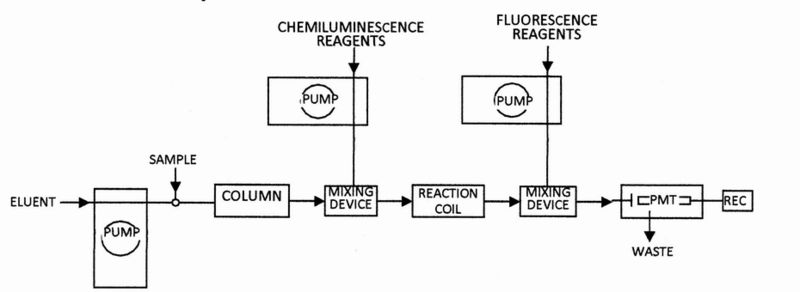
Figure D10.2 – Post-column instrumentation for measuring chemiluminescence after derivatization of analytes (PMT = photomultiplier tube; REC = recorder).
Interfaces between chromatography columns and chemiluminescence can become very complex. For example, peroxy-oxalate chemiluminescence is frequently coupled with HPLC. As it detects only fluorescent analytes (see chapter B5), successful detection depends on derivatization of the analytes eluted from the column before the addition of the chemiluminescence reagents. Figure D10.2 shows part of the post-column arrangements used for the determination of catecholamines by peroxy-oxalate chemiluminescence after reaction with ethylene diamine, which produces fluorescent derivatives.[2] For simplicity, the arrangements for different temperatures in different parts of the system have not been shown.
References
[edit | edit source]
Chemiluminescence detection in capillary electrophoresis
D11. Chemiluminescence detection in capillary electrophoresis
[edit | edit source]Capillary electrophoresis has outstanding resolving power for extremely small samples, but this poses a challenge for detectors. Compared with other candidates, chemiluminescence detection has the advantages of being highly sensitive and requiring inexpensive equipment of simple design. In addition it is not affected by the high voltage used in the separation system, a particular problem for electrochemical detection, which is also highly sensitive. Ultra-violet absorbance detectors also have low cost and are widely used, but narrow capillaries make it difficult to arrange for long enough optical path lengths. Laser induced fluorescence has high sensitivity, but the equipment is costly and pre- or post-column derivatization of nonfluorescent analytes is necessary.[1]

Figure D11.1 – On-column coaxial flow interface between capillary electrophoresis and chemiluminescence detection (adapted from reference D9.1).
As with HPLC, there is an inherent problem of compatibility between the conditions needed for separation and those needed for chemiluminescence. Additionally, there is a potential problem with the stability of chemiluminescence reagents. Both of these are addressed by using the post-column mode rather than pre-column. Post-column interfaces are devices for mixing the eluent with the chemiluminescence reagents and for this purpose designs may make use of merging flow, coaxial flow or reservoir mixing. Interfaces may also be classified as off-, on- or end-column, depending on the site of detection and on whether this is isolated from the high voltage supply used for capillary electrophoresis.
The simplest interface, off-column and merging flow, did not find widespread application. Buffer flowed from a reservoir through the separation capillary and merged with the reagent at a four-way connector at the end of the column. The outlet arm carried the mixture to the chemiluminescence reaction coil and flow cell adjacent to a photomultiplier, while the fourth arm connected through a semi-permeable membrane to a second buffer reservoir (containing the ground electrode) immediately downstream of the merging point. This arrangement isolates the high voltage from the detection zone.
In contrast, an on-column coaxial flow interface has proved to be effective for a large number of applications.[2] Figure D11.1 shows the detector is located at the capillary outlet tip, so detection is “on-column”. The ground electrode is located in the effluent reservoir so that detection takes place within the high voltage zone. The separation capillary outlet is inserted coaxially into the reaction tube, giving rise to minimum turbulence and reproducible mixing.
References
[edit | edit source]