A New Model of the Atom
| This book may contain original research or unverifiable material. You can help verify material by citing reliable sources, or by asking for assistance in the project reading room. You can participate at Wikiversity if you wish to publish new research or material that has not been verified by reliable sources yet. |
| A Wikibookian believes this page should be split into smaller pages with a narrower subtopic. You can help by splitting this big page into smaller ones. Please make sure to follow the naming policy. Dividing books into smaller sections can provide more focus and allow each one to do one thing well, which benefits everyone. |
| A reader requests expansion of this book to include more material. You can help by adding new material (learn how) or ask for assistance in the reading room. |
A New Visualization of Atomic Structure
[edit | edit source]Introduction
[edit | edit source]Most models of atomic structure are very difficult to comprehend, especially at introductory levels. This tool will attempt to illustrate a more visually intuitive atomic model enabling a deeper understanding of how nature works at it most fundamental level. This allows a much improved understanding of chemical bonding and the creation of molecules all with minimal violation to the theory of quantum mechanics (which is beyond the scope of this essay).
The Gossamer Nature of Matter
[edit | edit source]First, one must understand the vast emptiness of an atom. Visualize a propeller on the wing of an aircraft (or the blades of a fan). Let this propeller have two props (or fan blades), 180 degrees apart, with a diameter of 1 meter. Facing the spinning propeller one observes a circle of 360°. It is important to note that this circle is translucent such that objects, in the observer’s line of sight behind the propeller, can be seen. Dependent upon the speed of the propeller’s rotation and speed of a bullet, one could fire bullets through this translucent circle and toward targets beyond, sometimes hitting a propeller (or fan blade) and sometimes hitting a target.
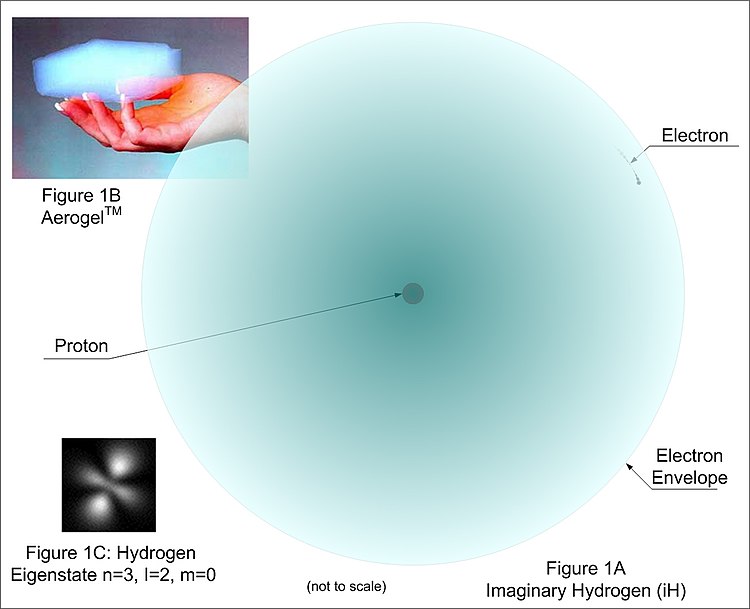
Keeping this translucent propeller in the mind’s eye, imagine having a magical BB, similar in size to that used in a pneumatic BB gun. For example’s sake, give this imaginary BB two attributes: 1) it must remain within a volume of a beach ball (say 1 meter in diameter), and 2) it must always move at nearly the velocity of light (as is predicted by modern sub-atomic theory). Think about what one would observe … an almost invisible 1 m diameter ball, yet due to the speed of the BB it would be very difficult to poke a stick through such a ball. Placing a hand against this sphere (and ignoring potential problems from friction) one would feel a solid surface (the outermost electron “shell”). Two such balls thrown at each other (or colliding) would mutually bounce away. But furthermore, they would have the additional force of mutual electronic repulsion powerful enough to prevent them from actually touching (unless enough pressure/heat is applied to cause bonding as explained later). A stadium could be filled with such imaginary spheres, one atop the other as a grocer stacks oranges, never violating each other’s space (never touching) and still the stadium would appear nearly empty. This mostly empty ball is very analogous to how the electron “cloud” of a hydrogen atom would appear at such a macro scale, except it would actually be much emptier, for if the hydrogen proton were the same size as this electron BB, the atomic diameter would be on a scale of kilometers . And actually, the commonly used term “cloud” is incorrect. Cloud, implies “many” as in fog with its countless aerosols. A better term is “envelope” or “orbital” as there is exactly one electron within the boundaries of its envelope. Let’s agree to refer to this imaginary electron particle as “iEp”, its envelope as “iEn”, and its velocity as “iEv”.
For reference, let us call this magic sphere “iH” for imaginary hydrogen. It is approximated in Figure 1A. Note the gossamer nature of the atom and that it is “see-thru”. So, it is much more accurate to visualize electrons as electron envelopes than as particles. A real-world visual analog is a substance called Aerogel® (Figure 1B; See also:Aerogel) that is 99% empty space! Note this is not like a gas or air. It is a state of matter with attributes that change with the number of protons, electrons and neutrons. To “poke” another electron envelope within a “shell” of electron envelopes requires specific conditions. These conditions vary with each element and current conditions such as heat and pressure. Furthermore, depicting the iEn as a sphere is a simplification. More accurate is to show the iEn as eigenstate orbitals. Figure 1C shows the eigenstates for iH where the probability of the electron’s position and velocity is proportional to the figure’s brightness. In actuality, the orbitals are in constant “rotation” about the nucleus. The sphere of Figure 1A is indicative of the boundary of rotation of these orbitals.
Now, imagine iH composing the earth’s entire atmosphere and the sun raining down visible iL, or particles of light (see below). Your mind’s eye should be able picture a thick blanket of mostly empty iH filled with the passing of billions of iL. With billions of iL passing through billions of iH, every once in a while an iL will hit an iH “just right”, scattering some of this imaginary light in this imaginary atmosphere. This is one way of understanding how different electromagnetic energy can pass through different materials. The iL with a frequency most easily scattered would give this imaginary atmosphere its imaginary color depending upon how may iL get through without hitting an iH electron or proton. If you have trouble “seeing” this then slow everything down and remember that if these figures were to scale then the orbitals and the space between each particle would be hundreds (even thousands) of times larger than can be shown on this page. Assume the iEp moves at 1 meter/minute such that it would take about 1 minute to traverse from one side of its envelope to the other. Next, imagine that iL travels at 1 meter/minute. As iL travels through all of these mostly empty iH in our imaginary atmosphere it would have to be at exactly the right time and place to strike either an electron or a nucleus.
A Conceptual Analogy for the Duality of Light
[edit | edit source]Next, we need imaginary electromagnetic light rays. It’s very difficult to understand the duality of light without mathematics. However, we may make an analogy that will allow one to “picture” how the photon (or electron) can be a wave and point particle simultaneously depending upon how it is observed. Additionally, this analog will allow one to grasp how it can have two types of spin. Let our imaginary photons be similar to the BB shown except they are squashed into a discus shape with the following attributes;
- 1) They move in straight lines (excluding gravitational effects) at the velocity of light,
- 2) They spin along their major axis at a frequency proportional to that of visible light, and
- 3) They spin along their minor axis.
Now we have a much more comprehensible tool for imagining the duality of light. Viewed from the side these moving particles will appear sinusoidal (similar to the “expanded iEp in Figure 2.), and viewed head-on they appear as a particle. Pick up a pencil, point its tip away from yourself, close one eye and use the other to view the eraser “head-on”. Imagine this was a fast spinning disk coming toward you. You should see a circle (looking like a particle), with no indication that it has a pencil trailing behind. Now, turn the pencil sideways, imagining it to be the path of the spinning disk, you should be able to imagine it as the passing of a sinusoidal wave. Thus, a spinning disk moving in a straight line may appear as either a particle or a wave simultaneously.
Let ”iL” designate such imaginary light particles. This concept of iL makes it much easier to understand how light acts, can have duality, and how it may be polarized by a filter. Assume a multitude of iL is directed at (shining on) a grate with separations smaller than the major diameter (Amajor) of iL but larger than iL’s minimum thickness (Aminor). Only those iL parallel to the grate would get through. Think of it as a discus spinning about both axes thrown towards a fence with picket gaps smaller than the discus diameter but larger than the discus is thick. A discus hitting the grate parallel to the ground will not get through the gaps, but those discus vertical to the ground as they near the grate will make it through the gaps. The passed iL would now be “in sync” or polarized.
Fault of polarizer analogy: A wire grid polarizer actually transmits the polarization that is spinning perpendicular to the grate's lines. In other words, the discus that gets through would actually be the one that 'whacks' the fence. If one considers the electric and magnetic fields of light as oscillating waves, the electrons in the conducting wires will respond in sync with the electric fields. The electrons moving freely along the axis of the wires will respond easily, and the light will be effectively absorbed and re-radiated within the material lattice, and mostly lost as Joule heating. The electrons will have very little freedom to move perpendicular to the wires however, and the impinging electric wave with perpendicular polarization will pass through mostly unaffected. [1] In fact, this is what is observed - light polarized perpendicular to the wires is transmitted.
A Mechanical Double-Slit Experiment
[edit | edit source]Using our imaginary moving discus model for light, one may explain the famous double-slit experiment in mechanical terms. In this experiment, a single beam of light passes through two very narrow slits to hit a wall. If light is a round particle one would expect it to show as two spots on the wall; But, it appears spread out along the wall primarily in three groups of concentration! How can this be? If we assume that light is a spinning discus we may explain that as the discus passes through the double-slit; it will be at various angles of rotation, or phase. The phase will determine which slit the discus passes, and if we imagine that it touches one side or the other of the slit as it passes, it will have a variation of deflection. As the discus particles careen through the slits, they may show up at any place along the wall, but when all the factors are considered (the probability of the outcome), the vast majority of the disciiTemplate:Fix/category[check spelling] will hit three places on the wall. These factors include: Double-slits to wall distance, width of slits, distance between slits, depth of each slit, and light particle/wave phase.
Helium
[edit | edit source]
What might imaginary helium (iHe) look like? The mass of iH is equal to the mass of one electron plus one proton while the mass of helium is equal to the mass of two electrons plus two protons plus two neutrons, or four times that of iH. But the shape of iHe is more like a dumbbell than a sphere. Figure 2 shows two protons and two neutrons at the center of opposing electronic envelopes. Due to like charges each electron has trouble violating the others envelope, and as they must maintain nearly the velocity of light, they seem to be everywhere in their own envelope at once. One must imagine these envelopes as three dimensional coming out of the page as well as below, more like an apple eaten down to the core!
Classical Model vs. New Model
[edit | edit source]
Figure 3A shows a typical middle school rendering of the carbon atom. Such a view is inaccurate and makes nuclear electro-mechanics more difficult to comprehend. The imaginary carbon (iC, Figure 3B) and imaginary oxygen (iO, Figure 3C) is more realistic and will lead to a much more intuitive understanding of chemical bonding (the dotted lines are for visual reference only). Performing the "thought experiment" of imagining the push-pull of electromagnetic forces upon these various particles, one can "see" how these subatomic components hold together. The two electrons in the innermost shell of Figure 3C are compelled to “fall” towards the nucleus, repel one another, and maintain near light speed. The four electrons in the outer shell are in a similar predicament, rapidly organization into synchronized equilibrium. This equilibrium is what defines substances and gives rise to their crystallization characteristics and chemical properties. Note that the Figure 3B model of carbon is an equilateral tetrahedron (a triangle in 3 dimensions). Triangles are among the strongest geometric forms, and equilateral triangles are the strongest triangles. Equilateral tetrahedrons are the strongest 3D shapes, and perfect for turning soft carbon or graphite to that hardest of natural substances... Diamonds! The more one learns about Nature, the more one can appreciate the connections from algebra to zoology. Nature can be wonderfully logical!
Chemical Bonding of Oxygen
[edit | edit source]
Noting that the space between all components of iO would be orders of magnitude greater than this page can show, Figure 4 shows schematically how two iO could bond. Assume that for whatever reasons that electron iO2e2 has come well into the influence of iO1e1 and iO1e2 as shown. We say they are sharing their shells. Since electrons repel each other electromagnetically atom iO1 and atom iO2 are now coupled together by the force labeled FR. Additional bonding force (FA) is supplied by the displaced of iO1e1 and iO1e2 being attracted to iO2’s nucleus. This is much like linking your knuckles together and attempting to pull your hands apart. But if electrons repel how could they get into such a configuration to begin with? Either by pressure & heat, or catalytically, or electromagnetically. If the pressure and heat is sufficient, the electron envelopes deform allowing the outer shells to slip amongst one another. This is how the sun and deep earth turn carbon to diamond. If a catalyst has the correct properties, it can pull the electron envelopes apart creating a “hole” for another envelope to slip into, freeing the catalyst to slip away for another reaction. This is how catalytic converters convert automobile emissions to less toxic substances. Electromagnetically, electron envelopes may be pulled apart creating a “hole” for coupling. This is how “buckyballs” are created in an electric arc.
Atomic Crystal Matrix
[edit | edit source]
This new schematic view of atomic structure lends itself well to showing how atoms form crystalline structures. Figure 5 shows such a matrix schematically (inner shell removed for clarity). Again, this is a 2-D analog of a 3-D structure, so one must imagine some of the electron envelopes going under the page while others extend above the page (the gray circles are for visual boundary reference only).
This model also explains the cohesion of water (H2O) and the release of energy when hydrogen is burned in the presence of oxygen. Figure 6A shows one oxygen and two hydrogen in close proximity (hydrogen shown with it’s single electron as four eisenstate orbitals). If enough energy is added, electron envelopes (or orbitals) can jump to a “higher shell”, creating room for hydrogen orbitals to “slip” between, such that hydrogen’s electron envelopes are “lower” than or within oxygen’s electron envelopes (Figure 6B).
The Combustion of Hydrogen and Oxygen
[edit | edit source]Burning into Water
[edit | edit source]
When oxygen releases the energy previously gained, its electron envelopes fall back to their “lower shell”, but now, all three atoms' mutually repellent electron forces “squeeze” the hydrogen envelopes releasing more energy (Figure 6C). Note, that the iEn volume of a free iH is much reduced. This reduction is a tremendous loss of energy. Think about this: If 2 volumes of iH are mixed with 1 volume of iO and ignited, a huge amount of energy is released and the resultant volume of water is much smaller than the original 3 volume units!
The Release of Energy
[edit | edit source]
Further note in Figure 7, how the attachment of 2 iH to one iO exposes naked protons. One may easily discern how such exposed protons would automatically adhere to any surface exposing electrons. Now one is able to visualize why H2O clings to a glass and why it is such an excellent solvent and reactant. With this model of the atom chemistry may now be visualized much more logically! A hydrophobic substance would have hydrogen trapped on its surface making it water repellent. Think of oil. They are often hydro-carbons strings of C+H2, with the hydrogen exposed such that oil and water don’t mix!
Electrons: Shells, Eigenstates & Orbits
[edit | edit source]Figure 8 presents a computer generated view of an element with many electrons. Note the “shells” are clearly delineated. The energy differential between each shell is a quantum unit and the movement from one shell to another is called a quantum jump. For an electron to jump from an inner shell to an outer shell it must absorb one quantum unit of energy per jump. Likewise to jump from an outer shell to an inner shell it must give up one quantum unit per jump, often in the form of a photon on light. Be aware that the distance between these subatomic components is several orders of magnitude larger than can be represented on a sheet of paper, such that the atom is much “emptier” than it looks. For example, if the nucleus contained protons and neutrons the size of BB’s (4.33mm) the innermost shell’s diameter would be approximately 200 meters; the second shell’s diameter 800 meters, and the third shell’s diameter 1900 meters. Thus, on the scale of BB’s, one would need a 2 kilometer square paper sheet to show Figure 8 at true scale! Emphasizing once again how “empty” atoms are. Amazingly, on a percentage basis, the nucleus is even emptier… but, that is a story for another paper!
Figure 8: See Dauger Research’s excellent Atom in a Box.
References
[edit | edit source]- ↑ “The Wire Grid Polarizer.” Optics, by Eugene Hecht, Pearson Education Limited, 2017, p. 347.