Structural Biochemistry/Organic Chemistry/Organic Functional Group/Aldehyde
Introduction
[edit | edit source]An Aldehyde is a major functional group in Biochemistry with the general formula, RCHO. This formula is made up of a carbonyl functional group where the two remaining bonds of the carbon are used to bond to a Hydrogen and an R funtional group which can be a second hydrogen, an alkyl or an aryl group. Due to the presence of the hydrogen on the central carbon Aldehydes, are by defintion located at the end of a carbon chain, unlike ketones that by definition are somewhere in the middle of the carbon chain.[1]
Nomenclature
[edit | edit source]IUPAC naming system requires the following steps in order to appropriately name molecules with aldehyde functional groups:
- Locate the longest carbon chain in the molecule that contains the aldehyde group and name it as if it were an alkane.
- Replace the -e ending of the alkane with -al
- Because aldehydes are always at the terminal end of the chain their carbonyl carbon is labeled 1
- Name the remaining functional groups in the chain
Common names for Aldehydes can be categorized into two separate groups. The first is a derivative of the name used for carboxylic acids such as formic acid (HCO2H) which is used to name the common aldehyde, formaldehyde (CH2O). The second group of commonly named aldehydes comes from flavorants such as 3-phenyl-2-propenal which is almost always referred to as cinnamaldehyde.[2]
Physical Properties of Aldehydes
[edit | edit source]
The carbonyl group within aldehydes creates a polar functional group with a dipole moment of approximately 2.5. The polarity allows for a resonance structure that shifts a pair of shared electrons between carbon and oxygen to the oxygen atoms resulting in a positively charged carbocation and a negatively charged oxygen atom as shown to the right. In addition to the stabilizing effect from this resonance, the lone pair on the oxygen of the carbonyl also acts as a hydrogen acceptor and therefore increases the solubility aldehydes when compared to hydrocarbons to 0.04g/100mL. The polarity of the aldehyde's carbonyl carbon allows for fast, endothermic addition of water and even faster, exothermic elimination of water. The presence of the oxygen in aldehydes increases the basicity of the group when compared with a regular alkene group. It is this additional basicity which lowers the transition state energy for these addition/elimination reactions to occur much more rapidly than for alkenes.[3]
Aldehyde Synthesis
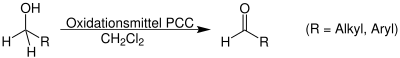
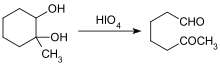
[edit | edit source]Aldehydes can be formed by several methods:
- Anti-Markovnikov hydration of alkynes
- Oxidation of 1° Alcohols
- Glycol Cleavage
- Ozonoloysis of Alkenes
Aldehydes act as a vital intermediate molecule for the assembly of more complex organic molecules.[4]


Roles of Aldehyde in Biochemistry
[edit | edit source]One of the major roles of Aldehydes in biochemistry is found within carbohydrates. Carbohydrates are the most abundant natural organic compound and are used by the body to store energy, found in cell walls to support cell structure, and make up a portion of the RNA and DNA backbones. Carbohydrates play key roles througout the body, for examply the immune system binds complement proteins to carbohydrates in order to signal the body to attack foreign cells. Glyceraldehyde and dihydroxyacetone are intermediates for glycolysis, D-Eurythrose is an intermediate in carbohydrate metabolism, D-Arabinose is presentin cell walls, L-Arabinose is present in plant glycoproteins, D-Galactose is found in the milk we drink, and so much more.[5]
- ↑ http://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/aldket1.htm
- ↑ http://science.marshall.edu/castella/chm204/chap14.pdf
- ↑ http://www.chemguide.co.uk/organicprops/carbonyls/background.html
- ↑ http://www.organic-reaction.com/synthetic-protocols/functionals-groups/aldehyde/
- ↑ Professor Viadiu-Lecture #9
