Structural Biochemistry/Krebs Cycle (Citric Acid cycle)
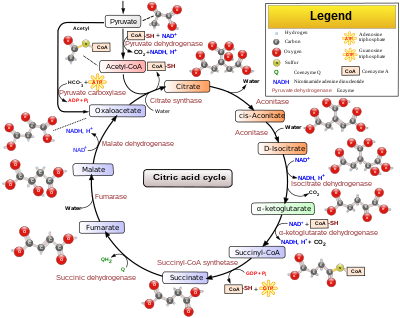
The Citric Acid Cycle has eight-steps.
Citric Acid Cycle
[edit | edit source]Other name for citric acid cycle is tricarboxylic acid (TCA) cycle or the Krebs cycle. The citric acid cycle is the central metabolic core of the cell. It is the final common pathway for oxidation — in other words harvesting high energy electrons--fuel molecules such as carbohydrate fatty acids, and amino acids by entering the cycle as Acetyl Coenzyme A (CoA). This reaction takes place inside of mitochondria. It is very efficient because it can generate large amounts of NADH and FADH. The citric acid cycle provides the majority, 90 percent, of energy used by aerobic human cells. By acting as the first stage of cellular respiration, the generation of high energy electrons from the citric acid cycle, in turn, are used in oxidative phosphorylation to reduce O2, generate proton gradient, and, later, the synthesis of ATP.
Citric Acid Cycle Links to Glycolysis by Pyruvate Dehydrogenase
[edit | edit source]Carbohydrates are mostly processed by glycolysis into pyruvate. Depending on the organism, the pyruvate is converted into either lactate or ethanol under anaerobic conditions. Through a specific carrier protein embedded in the mitochondrial membrane, pyruvate is transported into the mitochondria under aerobic conditions. Then, the pyruvate is oxidatively decarboxylated by the pyruvate dehydrogenase complex in order to form the acetyl CoA in the mitochondrial matrix. The reaction is shown in the following:
Pyruvate + CoA + NAD+ → acetyl CoA + CO2 + NADH + H+
This is an irreversible reaction which links glycolysis and the citric acid cycle together. As shown, CO2 is produced by pyruvate dehydrogenase complex and it captures high-transfer-potential electrons in the form of NADH. Therefore, the pyruvate dehydrogenase relates to the reactions of the citric acid cycle itself. The pyruvate dehydrogenase complex is composed of three different enzymes. Its complex is composed of members of a family of homologous complexes which include citric acid cycle enzyme a-ketoglutarate dehydrogenase complex. These complexes are very large, even bigger than ribosomes, with its molecular mass to be in between 4 million to 10 million daltons.
Reactions
[edit | edit source]- Acetyl-CoA, main product of the Pyruvate Dehydrogenase Complex in aerobic respiration, starts the Krebs cycle. (An irreversible reaction that is link between glycolysis and the citric acid cycle.) The mechanism of the synthesis of acetyl coenzyme A from pyruvate requires five coenzymes and three enzymes. It is a very complex mechanism which many enzymes and coenzymes. Cofactors, which function as substrates, are divided into two different cofactors which are catalytic cofactor and stoichiometric cofactor. The catalytic cofactor includes coenzymes such as thiamine pyrophosphate (TPP), lipoic acid, and FAD. The stoichiometric cofactor includes coenzymes such as CoA and NAD+. Pyruvate converts into acetyl CoA in three distinct steps which include: decarboxylation, oxidation, and transfer of the resultant acetyl group to CoA.
Pyruvate results in formation of acetyl-CoA by a three step reaction:
1. Decarboxylation: TPP is combined with the pyruvate and decarboxylated in order to yield hydroxyethyl-TPP. Of the pyruvate dehydrogenase component, TPP is known as the prosthetic group which the carbon atom between the nitrogen and sulfur atoms in the thizaole ring is more acidic than most double bonded carbon groups with pKa values near 10. This reaction is catalyzed by the (E1) pyruvate dehydrogenase component of the multienzyme complex. The carbon center located in the TPP is ionized to form a carbanion which is added to the carbonyl group of pyruvate. As part of decarboxylation, a positive charged ring of TPP stabilizes the negative charge which was transferred to the ring. Finally, the protonation yields hydroxyethyl-TPP.
Pyruvate + TPP( conenzyme thiamine pyrophosphate)+ 2 H+ --> Hydroxyehthyl-TPP + CO2
2. Oxidation: In order to form an acetyl group, the hydroxyethyl group which is attached to TTP is oxidized. Simultaneously, the hydroxyethyl group is transferred to lipoamide which is lipoic acid derived that links to the side chain of a lysine residue by an amide linkage. This creates the formation of an energy-rich thioester bond. In this reaction, the disulfide group of lipoamide acts as an oxidant and is reduced to the disulfhydryl form. This reaction is catalyzed by the pyruvate dehydrogenase component (E1) as well and yields the acetyllipoamide.
Hydroxyethyl-TPP + Lipoamide --> TPP + Acetyllipoamide
3. Formation of Acetyl CoA: Formation of Acetyl CoA: In this step, acetyl CoA is formed when acetyl group is transferred from acetyllipoamide. This reaction is catalyzed by dihydrolipoyl transacetylase (E2). As the acetyl group is transferred to the CoA, the energy-rich thioester bond is preserved. Thus, the fuel for the citric acid cycle, acetyl CoA has been generated from pyruvate for use. Until the dihydrolipoamide is oxidized to lipoamide, the pyruvate dehydrogenase complex cannot complete another catalytic cycle.
CoA + Acetyllipoamide --> Acetyl CoA + Dihydrolipoamide
4. Formation of NADH: The final step in this reaction occurs when the oxidized form of lipoamide is regenerated by dihydrolipoyl dehydrogenase (E3).Two electrons are transferred to first an FAD prosthetic group of the enzyme and then to NAD+. This process of transferring electron is very unusual because FAD are known to receive electrons from NADH, not transfer them. Within the enzyme, the electron-transfer potential of FAD is increased by its chemical environment which enables it to transfer electrons to NAD+. Flavoproteins are proteins which are tightly associated with FAD or FMN also known as flavin mononucleotide.
Dihydrolipoaminde + FAD --> Lipoamide + FADH2 + NAD+ --> FAD + NADH + H+
Overall reaction:
Pyruvate + CoA + NAD+ --> acetyl CoA + CO2 + NADH +H+
Citric Cycle Reactions
[edit | edit source]1. The first reaction of the cycle is condensation of acetyl-CoA with oxaloacetate to form citrate. In this reaction, acetyl group is joined to the carbonyl group of oxaloacetate. The reaction between acetyl-CoA with oxaloacetate is necessary to form an active site closed citryl CoA complex for hydrolysis because the active site of acetyl-CoA with hydrolysis is a wasteful process.
Oxaloacetate + Acteyl-CoA --> Citryl-CoA + H2O -->Citrate + CoA
2. Formation of Isocitrate via cis-Aconitate: Enzyme, aconitase catalyzes the reversible transformation of citrate to isocitrate. This is actually a 2 steps mechanism, which interchange an hydrogen with an hydroxyl group. First citrate is dehydrated to form the intermediary formation of the tricarboxylic acid cis-aconitate. Then by using the same enzyme, aconitase, isocitrate is formed. Aconitase is an iron-sulfur protein that participate in the dehydration and rehydration of the substrate.
Citrate <-(forward rxn removes water)-> cis-Aconitate <-(forward react with water)-> Isocitrate
3. Oxidation of ioscitrate with NAD+ to α-Ketoglutarate, CO2 and NADH. Isocitrate dehydrogenase catalyzed oxidative decarboxylation of isocitrate to form α-ketoglutarate. Mg2+ is used to interact with the carbonyl group of oxalosuccinate. The rate of formation of α-ketoglutarate determines of overall reaction in the citric cycle. The intermediate, oxalosuccinate, is a very unstable β-ketoacid. Reaction with Intermediate:
Isocitatrate + NAD+ --> Oxalosuccinate + NADH + H+ Oxalosuccinate + H+ --> CO2 + α-ketoglutarate
Overall Reaction:
Isocitrate + NAD+ --> α-ketoglutarate +CO2 + NADH
4. Oxidation of α-ketoglutarate to succinyl-CoA and CO2. This is another oxidative decarboxylation. Alpha-ketoglutarate is converted to succinyl-CoA and CO2 by the action of the α-ketoglutarate dehydrogenase complex. NAD+ is used as electron acceptor and CoA as the carrier of the succinyl group. This reaction is virtually identical to the pyruvate dehydrogenase reaction. It has three enzymes participating in this step. This is similar to the Pyruvate Dehdrogenase Complex reaction.
α-ketoglutarate+ CoA + NAD+ -(α-ketoglutarate dehydrogenase complex)-> succinyl-CoA + CO2A +NADH
5. Conversion of Succinyl-CoA to Succinate: Succinyl-CoA has a thioester bond. Thioester bond retains a lot of energy (ΔG° = -33.5 kJ mol -1. Energy is released by breaking the thioester bond. The enzyme that is used in this step is called succinyl-Co synthetase or succinic thiokinase. The cleavage of the bond is coupled with GDP getting phosphorylated.
Succinyl CoA + Pi + GDP --> succinate + CoA + GTP
6. Oxidation of succinate to fumarate: the succinate formed from succinyl-CoA is oxidized to fumarate by the enzyme called succinate dehydrogenase. FAD, which is attached to histidine side chain, acts as the electron acceptor by removing two hydrogen from succinate. FADH2 will pass its electrons to coenzyme Q, which will be use in the electron transport chain.
His-FAD + succinate <--> His- FADH2 + fumarate
7. Hydration of fumarate to malate: fumarate is then converted to malate by using fumarase. This enzyme is highly stereospecific; it catalyzes hydration of the trans double bond of fumarate. The reaction with add H+ and OH- to make L-malate.
Fumarate + H2O --> L-Malate
8. In the final step, Malate (C4), in a oxidation-reducation reaction catalyzed by malate dehydrogenase, is oxidized to oxaloacetate (C4) with NAD+ being reduced to NADH. The reaction is very positive with ΔG° = +29.7 kJ mol -1.
Malate + NAD+ <--> oxaloacetate + NADH + H+
9. Then, the Krebs cycle restarts again as long as Oxygen is transported into the cell.
Note that the number of Carbon is going from the sum of 4 and 2 from the beginning to 6, 6, 5, 4, 4, 4, and back to oxaloacetate (C4) again. Overall, there are 6 NADH, 2 FADH2, and 4 CO2 being produced by two acetyl-CoA molecules from the Pyruvate Dehydrogenase Complex.
Lipoamide Between Different Active Sites
[edit | edit source]Since all the complex pyruvate dehydrogenase structure is known, the atomic model was able to be formed so that its activity could be understood from it. The center of the complex is formed by the transacetylase component E2. Transacetylase contains eight catalytic trimers which are gathered to form a hollow cube. There are three major domains for each three subunits that form a trimer. The amino terminus has a small domain that contains a bound flexible lipoamide cofactor which is attached to a lysine residue. This domain is homologous to biotin-binding domains like pyruvate carboxylase. The lipoamide domain is followed by a small domain that interacts with E3 within the complex and the larger transacetylase domain completes an E2 subunit. E1 is considered to be α2β2 tetramer, and E3 is considered to be an αβ dimer. E2 is surrounded by multiple copies of E1 and E3. These three distinct active sites work together through the long, flexible lipoamide arm of the E2 subunit that carries substrates from one active site to the other. The process of moving lipoamide between different active sites are:
1) In the active site of E1, pyruvate is decarboxylated and forms a hydroxyethyl-TPP intermediate while the CO2 leaves as the first product. The active site is connected to the enzyme’s surface through a long hydrophobic channel within the E1 complex.
2) The lipoamide arm of the lipoamide is inserted by E2 into the deep channel in E1 which leads to the active site.
3) The transfer of the acetyl group is catalyzed by E1 to the lipoamide. The acetylated arm leaves E1 and enters the E2 cube in order to visit the active site of E2. This is located deep in the cube at the subunit interface.
4) The acetyl moiety is then transferred to CoA. The second product which is the acetyl CoA, leaves the cube. The reduced lipoamide arm then swings to the active site of the E3 flavoprotein.
5) The lipoamide is oxidized by the coenzyme FAD in the E3 active site. The reactivated lipoamide is ready to start a new reaction cycle.
6) NADH, the final product, is produced through the reoxidation of FADH2 to FAD.
- This coordinated catalysis of a complex reaction is possible because of the structural integration of three different kinds of enzymes and the long, flexible lipoamide arm. The overall reaction rate is increased and the side reaction is minimized due to the proximity of one enzyme to another. Throughout the reaction sequence, all the intermediates in the oxidative decarboxylation of pyruvate remain bound to the complex. This is readily transferred to the flexible arm of E2 calls on each active site in turn.
Pyruvate Dehydrogenase Complex
[edit | edit source]Although glucose can be formed by pyruvate, the irreversible step of the formation of acetyl CoA from pyruvate causes the acetyl CoA to be unable to convert back into glucose. The oxidative decarboxylation of pyruvate to acetyl CoA commits the carbon atoms of glucose to one of two principal fates: oxidation to CO2 by the citric acid cycle, with the concomitant generation of energy, or incorporation into lipid. The activity of the pyruvate dehydrogenase complex is stringently controlled. The reaction can be inhibited by the high concentration of reactions: by binding directly, the acetyl CoA inhibits the transacetylase component E2 while the NADH inhibits the dihydrolipoyl dehydrogenase E3. High concentrations of NADH and acetyl CoA inform the enzyme that the energy needs of the cell have been met or in order to produce the acetyl CoA and NADH, the fatty acids are being degraded to produce because most pyruvate is derived from glucose by glycolysis. Covalent modification is very important in regulating the complex in eukaryotes. The phosphorylation of the of the pyruvate dehydrogenase component (E1) by pyruvate dehydrogenase kinase I (PDK) switches of the activity of the complex. The deactivation is reversed by the pyruvate dehydrogenase phosphate.
Regulatory Enzyme in Citric Acid Cycle
[edit | edit source]In animal cells, the rate of citric acid cycle is regulated to fitted the required needs for ATP. The two enzymes that allosteric control the cycle are isocitrate dehydrogenase and α-ketoglutarate dehydrogenase, which both generate high-energy electrons in cycle.
Isocitrate dehydrogenase is stimulated by ADP that increase the affinity of enzyme for substrates. Since isocitrate, NAD+, Mg+, and ADP are cooperative, the binding of these substrates are regulated. If energy is unnecessary, there will be more NADH and ATP that will compete for the binding of enzyme with NAD+ and ADP respectively. Because the reaction requires positive energy or electron acceptors, the reactions is slowed until high energy like ATP and NADH are needed.
α-ketoglutarate dehydrogenase is another allosteric enzyme that regulated the rate of citric acid cycle for ATP. This enzyme is inhibited by succinyl CoA and NADH, which are the product that also compete for binding with reactants. α-ketoglutarate is also inhibited by high energy electron.
