Structural Biochemistry/Lipids/Membrane Transport
All cells need to acquire the molecules and ions that they need from their surrounding extracellular fluid. There exists an exchange of molecules and ions in and out of the cell wall, as well as in and out of membrane-bounded intracellular compartments such as the nucleus, ER, and mitrochondria. Examples of substances that exchange through the membrane include glucose, Na2+, and Ca2+, ATP, mRNA, etc. The lipid bilayer of cell membranes is impermeable to large and polar molecules but permeable to water molecules and other small uncharged molecules like O2 and CO2. To solve this problem, the cell membrane contains proteins that are selective for unique, water soluble molecules. Continuous protein pathways are composed of carrier proteins, channels and pumps. The transport may be active transport by carrier proteins with an energy source, or it may be facilitated diffusion or passive transport via channels. A cell's activity depends on what biochemical reactions it may complete, and these reactions are dependent on the compounds extracted from the extracellular fluid. The array of transporters expressed in any given cell defines the cell's function and effectiveness.
Quantifying Free Energy Stored In Concentration Gradients
[edit | edit source]The process or movement of any molecule or ion moving down or up a concentration gradient requires a change in free energy. Understanding free energy is the heart of understanding how molecules are transported and/or behave in a concentration gradient. From the second law of thermodynamics molecules spontaneously move from a higher concentration to lower concentration. The availability of free energy is one of the factors that determine if a molecule will move across a membrane, the other being the permeability of the molecule in the lipid bilayer. The free energy change in the transportation of an uncharged species from one side of a membrane containing a concentration of x1, to another side of a membrane containing a concentration of x2 is given as followed:
ΔG (x) = RT ln (x2/x1) = 2.303 RT log10 (x2/x1)
R: The gas constant, 8.314 x 10−3 kJ mol−1 K−1
T: Temperature
x: Concentration
ΔG: Free Energy
At 25° (298K), ΔG is +11.4 kJ mol−1 (+2.7 kcal mol−1), indicating that this transport process requires an input of energy.
For charged species, an electrical potential is generated by an unequal distribution of ion charges across the membrane because “like” charges will be repelled. Taking the sum of the electrical terms and the concentration, electrical potential, generates the general expression.
ΔG (x) = RT ln (x1/x2) + Ζ F ΔV = 2.303RT log10 (x2/x1) + Ζ F ΔV
Ζ: Electrical charge of the transported species.
F: Faraday’s constant 96.5 kJ V−1 mol−1.
ΔV: Potential in volts across the membrane.
When ΔG is positive the transport is active, an input of energy is needed to move a molecule up a concentration gradient, contrary to ΔG being negative the transport is passive, which means that such molecules will pass through a membrane down their own gradient, simple diffusion.
Passive Transport
[edit | edit source]
Passive transport is the moving of biochemicals across membranes of cells without the use of chemical energy. The four main types in include diffusion, facilitated diffusion, filtration and osmosis.
Diffusion is the process by which molecules migrate over the cell membrane from areas of higher concentration to areas of lower concentration. When the amounts of molecules become stabilized, this state is called equilibrium. This occurs through random molecular motion.
A diffusion coefficient is a factor of proportionality that represents the amount of substance diffusion across a unit area through a unit concentration gradient in unit time. It is represented with a D and is based on Fick's law of diffusion.
There are 2 kinds of diffusion:
1) Tracer diffusion is the spontaneous mixing of molecules that occurs in absence of a concentration gradient. This diffusion takes place under equilibrium
2) Chemical diffusion occurs in the presence of a concentration gradient. It will result in a net transport of mass. The overall system will increase in entropy and it gradually moves closer to equilibrium until it is reached.
Other types of diffusion include:
- Osmosis is the diffusion of water through a semi-permeable membrane. Water will move from an area with a higher concentration of water to the other side of the membrane with a lower concentration of water. Osmosis is very important in biological systems because many membranes are semipermeable. They are impermeable to organic solutes (i.e. large molecules) but are permeable to water and small uncharged solutes. Permeability does not only depend on size, but also depends on solubility properties, charge and chemistry.Further information: [1]
- Atomic diffusion is a diffusion process that incorporates the random thermally-activated movement of atoms in a sold, which eventually results in the net transport of atoms. Examples include how a Helium atom inside a balloon can diffuse through the wall of the balloon. The balloon will eventually deflate.
- Brownian motion is the random movement of particles suspended in a fluid (could be liquid or gas). There is also a mathematical model that is used to describe such random movements.
- Collective diffusion is the diffusion of a large number of particles. This usually takes place within a solvent. This is different form the brownian motion because it is the diffusion of a single particle and the interactions between the particles may need to be considered.
- Eddy diffusion is also called "turbulent diffusion." It is any diffusion process by which substances are mixed in the atmosphere or in any fluid system due to eddy motion.
- Effusion is the process in which individual molecules will flow through a hole without collisions between molecules. It occurs if the diameter of the hole is considerably smaller than the mean free path of the molecules.
Types of Passive Membrane Transport
[edit | edit source]Ion Channels
[edit | edit source]Ion channels are proteins that forms water-filled pores through membranes, which allow specific ions to passively transport down the electrochemical gradient. For instance, the ion channels commonly found throughout the human body include Na+ channels, K+ channels, Ca2+ channels, and Cl- channels. These passive transport ion channels make use of the electrochemical potential to drive physiological processes, such as nerve impulse. The electrochemical potential is maintained by various active transport mechanisms, such as the Na+-K+ ATPase.
The selectivity of the ion channels arise from the amino acids formation from which the protein is formed. Two main criteria determine what ions pass through these channels unimpeded: diameter of the pore and the electrical properties of the amino acids. The ion channels essentially forms a hole through the lipid bilayer of the membrane. The diameter of the hole helps determine which substances are allowed to pass through the channel. Only ions of an appropriate size will be able to pass through the hole of a specific ion channel. The observation that some larger ion channels only permit a specific ion to pass while smaller ions do not is based on the energetics of dehydrating the ion as it passes. The arrangement of amino acid carbonyl groups inside channels dictate the passage: the arrangement of the carbonyl groups is so that the dehydration energy costs are lowest for certain hydrated ions. Thus a larger pore has a favorable arrangement for larger hydrated ions, whereas a smaller hydrated ion passing through would require more energy to dehydrate. In potassium channels, this dehydration region that mediates the passage of K+ ions is known as the selectivity filter.
In addition to the diameter of the pore, the electrical properties of the amino acid help determine which ions are allowed to pass through the ion channel. If the amino acids facing the inside of the pore are negatively charged, then only cations can pass through the channel. Anions will be repelled away from the negatively charged amino acids in the channel due to charge repulsion. Conversely, anions will be able to pass through channels lined with positively charged amino acids.
There are some inherited ion-channel diseases. Some include: -Chloride-channel diseases include cystic fibroses and inherited tendency to kidney stones. -Potassium-channel diseases include some inherited life-threatening defects in the heartbeat, a rare inherited tendency to epileptic seizures in the newborn and several types of inherited deafness. -Sodium-channel diseases include inherited tendency to certain types of muscle spasms and Liddle's syndrome.
Porins
[edit | edit source]
Porins are a type of membrane protein that forms a beta-barrel pore across the lipid bilayer. Unlike other protein transport channels, the pores from porins are large enough to allow passive diffusion. As a result of the large diameter, porins generally mediate the diffusion of small metabolites, such as amino acids, sugars, and ions.
The general structural properties of various porins are the same, regardless of their type. Porins are proteins composed primarily of beta-sheets, connected in the anti-parallel direction. Sixteen to eighteen strands of beta-sheets fold into a cylindrical tube, which is labeled as the beta-barrel. The amino acid sequence is roughly an alternating pattern of non-polar and polar residues, which are positioned in an appropriate way as to generate the tertiary barrel structure. This beta-barrel is located in the lipid bilayer, forming the large hole into the cell. As a result of these interactions, the amino acids facing the outside of the beta-barrel are generally nonpolar, to interact with nonpolar region of the lipid bilayer. However, the inside of the beta-barrel typically contains polar amino acids, to interact with the aqueous environment connecting the two sides of the membrane.
Porins are also found in the outer membrane of gram negative bacteria. Gram negative bacteria contain an outer membrane that helps keep out unwanted chemicals, as well as increase virulence. The porins facilitate the diffusion of small molecules and nutrients through the outer membrane and into the periplasm.
Ionophores
[edit | edit source]Ionophores are molecules that help transport ions from a hydrophilic environment into a hydrophobic environment. In other words, ionophores are ion carriers that help transport hydrophilic ions across lipid bilayer membranes. There are two broad mechanisms by which ionophores transport ions across cell membranes: carrier and channel forming.
In the carrier mechanism, the ionophore forms a complex with the ion. The ionophore wraps around the ion with its polar interior. The exterior of the ionophore-ion complex is primarily hydrophobic, thus allowing the complex to cross the hydrophobic cell membrane. The carrier ionophore basically shields the ion’s charge from the environment by solvation.
For the channel forming mechanism, the ionophore induces a hydrophilic channel through the lipid bilayer membrane. The formation of this polar pore allows ions to cross through the cell membrane. Ionophores can be used as antibiotics due to its ability to disrupt the transmembrane electrochemical gradient. This electrochemical gradient is essential in driving metabolic processes. Without the gradient, there would be no net movement of ions into and out of the cell, essentially disrupting normal cellular processes. Ionophores disrupt the electrochemical gradient by allowing ions to freely diffuse across the cell membrane, either by forming complexes or channels. This free diffusion disrupts the normal ion balance between cytoplasm and the extracellular environment, thus eliminating the electrochemical gradient.
Facilitated diffusion
[edit | edit source]Facilitated diffusion occurs in almost all cells. Facillated diffusion of ions takes place through proteins or assemblies of proteins that are embedded in the plasma membrane. These proteins are water-filled channels through which ions can pass down their concentration gradient. These channels can be either opened or closed, and others can be considered "gated".
One critical example is the metabolism of glucose. Glucose molecules cannot pass easily through cell membranes; passive diffusion alone is slow, which can be problematic for cell metabolism. One way nature has solved this problem is by facilitated diffusion (another rapid mode of glucose transport is active diffusion). Like passive diffusion, the movement of glucose is always from a region of high to low glucose concentration and independent of ion coupling. Glucose transport proteins catalyze the reaction of glucose movement across the membrane. Facilitated diffusion of glucose molecules is fast and bidirectional. The actions of glucose transport proteins are analogous to enzyme mediated catalysis. [2]
Facilitated diffusion is a form of passive transport that uses no energy for the transport of molecules and substances through the membranes of cells. It is largely aided by proteins that are an integral part of the membrane. Facilitated diffusion is the spontaneous passage of substances or molecules across a membrane passing through specific transmembrane transport proteins. The hydrophilic substances can avoid contact with the lipid by layer core of the membrane by passing through transport proteins. Some of these proteins function by having a hydrophilic channel that lets through polar molecules. Aquaporins are an example of channel proteins that facilitates the transport of water molecules through the membranes. Other proteins termed carrier proteins grab the molecules and change shapes so as to avoid contact of the molecules with the hydrophobic core and goes through the membrane. Most of the proteins are very specific that is they only transport certain types of substances.
Gated Ion Channels
[edit | edit source]Ligand-gated
These ion channels open or close in response to when it binds to a signal molecule or "ligand." The ligand is not the substance that is transported when the channel opens. Some ions are gated by extracellular ligands and others are by intracellular ligands.
Another type of protein channels are ion channels that lets through ions. Many of them are stimulated by electrical or chemical signals and are called gated channels. The stimulants either open the channel or clothe them. Stimulation of a nerve cell by certain neurotransmitter molecules, for example, opens gated channels that allow sodium ions into the cell. External ligands bind to a site on the extracellular side of the channel. Examples include:
- Acetylcholine (Ach) which is the binding of the neurotransmitter acetylcholine at certain synapses. It opens channels that admit sodium ions and initiate a nerve impulse or muscle contraction.
- Gamma amino butyric acid (GABA)- binding of GABA at certain synapses allows for the central nervous system to admit chlorine ions into the cell, which inhibits the creation of a nerve impulse.
- Calcium channels that permit calcium ions to flow into the membrane. These Ca2+ ions then serve as second messenger internal ligands to initiate various processes, such as activating calmodulin.
External ligands may be regulated by enzymes. In neurosynapses, acetylcholinesterase degrades ACh into recyclable components; GABA is degraded in a similar fashion. Ca2+ is removed by active transport via an Na+/Ca2+ pump. These various ligand removal processes help regulate the overall concentrations and restore membrane asymmetry.
Internal ligands bind to a site on the intracellular side of the channel exposed to the cytosol. Examples include:
- cyclic AMP (cAMP) ad cyclic GMP (cGMP) which are both called "second messengers". They are channels that are involved in the initiation of impulses in neurons responding to odors and light
The channel that allows chlorine and bicarbonate ions in and out of the cell is regulated by ATP. This channel is defective in patients with cystic fibrosis
Mechanically-gated ion channels
- Sound waves bending the cilia-like projections on the hair cells of the inner ear. They open up, which leads to the creation of nerve impulses that the brain interprets as sound.
- Mechanical deformation of the cells of stretch receptors open up ion channels leading the to creation of nerve impulses.
Voltage-gated channels
These neurons and muscle cells are "excitable cells". Some channels open or close as a response to changes in the charge (measured in volts) across the plasma membrane. An example is an impulse that passes down a neuron and the reduction in the voltage opens sodium channels in the adjacent portion of the membrane.
Carrier Proteins
[edit | edit source]Carrier proteins are membrane-bound transport proteins that bind with specific substrates and changes conformation to carry the substrate across the membrane. Facilitated diffusion occurs by use of carrier proteins. There are several types of carrier proteins: uniport carriers, symport carriers, and antiport carriers.
Uniport carriers are carrier proteins that move only one kind of substance across the cell membrane. They work by binding to one molecule at a time and transporting that molecule down its electrochemical gradient. Uniport carriers can be regulated by various mechanisms: voltage, physical, and ligand binding. By the voltage regulation, the uniport carrier opens or closes at a critical difference between transmembrane voltage. For the physical regulation, physical pressure will cause the carrier to open or close. Lastly, for ligand binding regulation, ligands bind to the uniport carrier on either the intracellular or extracellular side to induce opening or closing of the carrier.
Other carrier proteins can carry more than one molecule across the cell membrane at a time. Symport carriers are carrier proteins that transport two or more different molecules simultaneously across the cell membrane in the same direction. In contrast, antiport carriers are carrier proteins that transport two or more different molecules simultaneously across the cell membrane, of which at least one of the molecules are transported in the opposite direction compared to the others. Generally, these carriers transport at least one molecule down its electrochemical gradient and the other molecules would go against its gradient. The molecules going down the electrochemical gradient will provide the driving potential to push the other molecules against its electrochemical gradient. These types of proteins are classified as secondary transporters, or cotransporters.

Facilitated diffusion by carrier protein transport is ultimately based on the embedded membrane proteins, in contrast with passive diffusion which occurs everywhere in the membrane. In particular, uniport transport and passive diffusion, both of which describe ion movement down concentration gradients across membranes, differ by their maximum rates: uniport transport is limited to and located at the carrier proteins in the membrane.
Active Transport
[edit | edit source]Active transport in a cell occurs when energy is used to transport molecules across the cell membrane. While passive transport takes advantage of favorable concentration gradients to facilitate ion diffusion through membranes (i.e. moving a protein from high to low concentration), active transport requires energy input because the molecule in question is being moved against the concentration gradient (i.e. moving a protein from low to high concentration). Because this type of movement is "uphill", meaning that it is thermodynamically unfavorable, energy is needed to compensate for the thermodynamic loss. This ensures that the transport in question is completed successfully and that the cell can obtain whatever nutrients it requires, even if this means moving proteins and ions into areas where their concentration is already relatively high. The ATP used in active transportation may be used either directly or indirectly. For direct active transport, some transporters will bind ATP directly and use the energy of its hydrolysis to drive active transport and establishes a concentration gradient. Indirect Active transport will use the energy already stored in the gradient of a directly-pumped ion.
Membrane Pumps
[edit | edit source]The cell makes use of membrane pumps to help in accomplishing active transport. Pumps can convert free energy into different forms, depending on which form is required by the cell at a given time. This property makes membrane pumps a convenient choice for mediating active transport since they can provide the energy needed to initiate the transport. The two main types of pumps employed by the cell are P-type ATPases and ATP-binding cassette transporters (ABCs). Both of these pumps are powered by ATP, one of the more common forms of cellular energy. One method by which these pumps can perform active transport is by binding to ATP. This binding, followed by hydrolysis, induces a conformational change in the pump that allows bound ions to be transported across the cell membrane. These pumps can also use active transport to establish favorable concentration gradients for separate transport processes. For example, one pump can create a given concentration gradient by performing active transport on a certain ion, and then another pump can exploit this new concentration gradient by facilitating ion diffusion down the concentration gradient. Thus the cell can couple active transport with passive transport (much like it couples endergonic reactions with exergonic reactions) in order to efficiently use the results of one process to drive another process to completion.
Na+-K+ pump
[edit | edit source]
The Na+-K+ pump, also known as the Na+-K+ ATPase, is an enzyme used by the cell to control the ion gradients in its intracellular media. As the name of the enzyme suggests, the ions it deals with are those of potassium and sodium, which are two of the more common ions present in living systems. In this case, the Na+-K+ pump hydrolyzes ATP in order to supply the energy needed to actively transport Na+ out of the cell while bringing K+ into the cell. It is for this reason that most animal cells tend to have a significantly higher concentration of potassium ions than sodium ions; the cell requires this concentration in order to be able to facilitate various cellular processes. The Na+-K+ pump helps it maintain this concentration by generating the necessary ion gradient.http://www.youtube.com/watch?v=bGJIvEb6x6w&feature=related
Two other such enzyme "pumps" that are homologs of the Na+-K+ pump are the Ca2+ ATPase and the H+-K+ ATPase. Ca2+ ATPase is responsible for transporting calcium ions (Ca2+) from the cytoplasm of normal tissue cells into the sarcoplasmic reticulum of muscle cells. Calcium ions are needed by the muscle cells in order to function at peak efficiency; thus this pump ensures that they receive enough calcium ions to fulfill this requirement. H+-K+ ATPase pumps large quantities of protons (H+) into the stomach's gastric juices in order to keep the pH less than 1.0. Such a low pH is required because the stomach is responsible for digesting all the foods and fluids that enter it; thus its gastric juices need to be acidic enough to dissolve and break down anything that needs digesting.
The three aforementioned pumps are all part of a family known as the P-type ATPases, so called because they all form a phosphorylated intermediate during their reactions with ATP. Hundreds upon hundreds of known homologs of these pumps exist within the P-type ATPases, and each plays a definite role in maintaining the functions of the cell.
Multidrug Resistant (MDR) Pumps
[edit | edit source]MDR Pumps are activated by ATP and are found in microorganisms such as bacteria and cancer cells. The multidrug-resistance (MDR) pumps consist of large proteins that weave through the cell-surface membranes. They work to effectively monitor and prevent unwanted chemicals from entering the cell. Therefore, the microbes have ability to self-defend with MDR pumps. MDR pumps also prevent antibiotics from entering bacteria and chemotherapeutic agents from entering cancer cells. In addition, they are used to spit out the ones that might endanger the bacteria. Administering antibiotics with MDR inhibitors may be a way to circumvent the MDR pumps in bacteria. This explains the uncanny ability of bacteria to defend themselves against antibiotics. MDR pumps can be found in humans, where they have variety of roles that help drugs get to places they need to go. They are also important in membranes in the brain, digestive tract, liver, and kidneys because they move hormones into and out of cells through the membrane (Medicines by Design 15).
For instance, AcrB is a effective MDR in Gram-negative bacteria. It functions through its three asymmetric protomers, each of which exists as a particular conformation that corresponds to its function in the pump. The three stages are Access, Binding, and Extrusion. In the Access stage, substrates enter the protomer's vestibule, with the binding pocket still left intact. In the Binding stage, the substrate remains in the vestibule, but the binding pocket expands to better hold the substrate. Lastly, in the Extrusion stage, the substrate exits via the removal of the central helix. AcrB serves to export a multitude of drugs out of the membrane, some of which include antiseptics, toxic compounds, and antibiotics.
Vesicles
[edit | edit source]Vesicles are simply a bubble of fluid within a cell. Specifically, vesicles are a membrane bound sac that aid in transportation and storage of cellular waste or cellular products, metabolism, and buoyancy control. The membrane surrounding the vesicles shares many characteristics with the plasma membrane of the cell. As a result, the vesicle can fuse with the membrane in order to dispose of its contents.
Transport Vesicles
These are just a specific type of vesicle with a function of transport. They move molecules, products etc. between the Rough Endoplasmic Reticulum and the Golgi apparatus. Two types of proteins are synthesized on the ribosomes present on the Rough ER. From here the transport vesicles take the new proteins to the Golgi apparatus. At the Golgi apparatus the proteins age and mature in order to be transported to their final destination. The proteins always travel around the inside of the cell with transport vesicles.
Lipid Vesicles
Also known as liposomes, these vesicles are aqueous compartments that are surrounded by a lipid bilayer. These vesicles are used to monitor membrane permeability and to deliver chemicals to the cell. Lipid vesicles aid in determining the level of impermeability of a membrane to ions and polar molecules. Ions and polar molecules are trapped in the aqueous compartment of the liposome. The rate of the flow from the inner compartment of the vesicle to the outside solution during membrane transport determines the membrane's impermeability to the ions or polar molecules contained in the inner aqueous compartment. Liposomes are formed by sonicating phospholipids in the presence of an ion, polar molecules or a water soluble substance.
Coated Vesicles:
These are vesicles that form when emerging buds are detached from the cell membrane. Depending on the formation of the vesicle, the formation of the outer coat may form the vesicle, where in other cases, other enzymes are necessary to form the vesicle. It is an intracellular structure. The outer surface of the vesicle is covered in a lattice or cage like coating of the protein Clathrin.
Clathrin is a protein that forms a lattice shaped covering on the cytosolic side of the cellular membrane. These cytosolic sides are referred to as coated pits, the first stage of forming coated vesicles. It is made through a process of making subunits called triskelioins. The Triskelioins are a three pronged molecule with three N-terminus regions. They are known as heavy chains, about 192kDa in weight and are bound to light chains, about 30kDa in weight. The invagination of the pit is during the first states of endocytosis and results in a clathrin coated vesicle. Clathrin can self assemble spontaneously and it plays a major role in deforming the budding vesicle. While clathrin is a major player in the deformation, there are other accessory proteins that aid in the assembly and dissembly of the coats.
COP I is another protein that coats vesicles during protein transportation. However, this vesicle coat transports from the Golgi apparatus to the Rough Endoplasm Reticulum. Since this is in the reverse of direction of normal transportation, it is known as a retrograde transport. The protein coat is made of large protein sub-complexes that possess 7 unique subunits.
The protein is known as an ADP Ribosylation Factor dependent adaptor protein. This basically means that they are regulators of vesicle biogenesis. They also aid in the selection of the proteins that are used for the carriers.
COP II is very similar to COP I in the fact that it transports proteins, however COP II transports from the Rough ER to the Golgi apparatus and is known anterograde transport. COP II has a coat that is made of 4 unique protein subunits. COP II has three different binding sites that it can complex with other proteins.
Both COP I and COP II are very active in membrane trafficking. They perform the necessary tasks of selecting the correct cargo for the proteins and they change the shape of the phospholipid bilayer into the correct buds and vesicles.
The anterograde and retrograde transport off set each other to help keep the flow of the membranes and secretions in balance. There is constant recycling going on to maintain the equal amounts of resident proteins in the different pathways.
Exciting the Membrane
[edit | edit source]Semipermeable membranes are often sensitive to charge and voltage across the lipid bilayer. Remember, membranes are charged entities so exacting a charge across a membrane is likely to cause a reaction such as an action potential. An action potential is generally created at the axon hillock once the membrane is depolarized due to an increase or decrease of voltage. When the membrane potential goes up, integral proteins in the membrane called voltage gated ion channels start to open to allow ions into the cell. While some ion channels allow ions into the cell, other channels let ions out of the cell. This is most exemplified by the sodium potassium pump. So since sodium ions are flowing into the cell and potassium is flowing out of the cell, depolarization occurs where the usual membrane potential, called the resting potential is disturbed. Note that action potentials are all or nothing, meaning the occurrence of action potentials does not depend on the magnitude of stimulus. A single action potential can trigger several along the membrane thus propagating the signal.
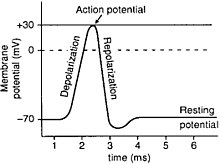

Only certain types of cells have the activity of action potentials. The most important one is the neuron. A neuron is a cell in the nervous system that creates messages and sends signals through electronic excitability through action potentials. The axon hillock is the part of the neuron that connects a cell to the axon. This area has many voltage activated ion channels and is considered to be the key ingredient to creating the depolarization that exacts an action potential.

Neurotransmitters are chemicals that amplify or control the signal between a neuron and another cell. When neurotransmitters are released into the synaptic cleft, they bind to receptors and cause an action potential which can then trigger other neurons to do the same creating a surge of energy across a group of neurons that carry out a specific process. This overall process is called neurotransmission. Action potentials can be transferred from one cell to an adjacent cell because of direct connection of these cells through gap junctions. Channels and other membrane proteins make sure that action potentials only travel one direction. Some cells need no stimulus to fire action potentials while others need an external stimulus. For example, the pacemaker cells in the heart maintain a rhythm that can be changed and altered with external stimuli such as a shock to the body. While sensory neurons like the ones in your eyes that sense light are excited by external source which then provokes action potentials. This is the same for cells in the ear that are sensitive to sound.
Several chemicals block neurotransmitter binding by competitively binding to the receptors. Substances such as curare or atropine, dubbed antagonists, compete with neurotransmitters, specifically acetylcholine (ACh), binding to and preventing the ligand-gated channels from opening and allowing ions to flow, thus preventing the generation of action potentials. In a natural environment, this competitive inhibition leads to paralysis and death. In a controlled environment, these antagonists can be used to prevent muscle spasms or convulsions during surgery.
References
[edit | edit source]Alberts, B., Bray, D., Lewis, J., Raff, M. and Roberts, K. 1989. The Cell, 2nd Ed.
Berg, J.M, Tymoczko, J.L. and Stryer, L. Biochemistry. 6th ed. New York: W.H. Freeman and Company, 2007. Print.
Carruthers, Anthony. 1990. “Facilitated diffusion of glucose.” Physiological Review, vol 70-4: 1135-76. PubMedhost(accessed November 16, 2009).
Silverthorn, Human Physiology 5th Edition
Purves et al., Neuroscience, 4th Edition
http://www.biologie.uni-hamburg.de/lehre/bza/kanal/porin/eporin.htm#
http://www.answers.com/topic/ionophore
http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mcb&part=A4038
Lodish, Harvey, et al. 2003. Molecular Cell Biology 5th Edition. pages 708-710. W. H. Freeman.
http://en.wikipedia.org/wiki/ATP-binding_cassette_transporter#Mechanism_of_Transport

