Introduction to Inorganic Chemistry/Acid-Base Chemistry
Chapter 3: Acid-Base Chemistry
[edit | edit source]

Acids and bases are important for a number reasons in inorganic chemistry.
- Many industrially useful catalytic reactions involve inorganic acids and superacids, such as zeolites, anhydrous hydrogen fluoride, and sulfated zirconia. These acids are sufficiently strong in anhydrous media that they can protonate olefins and alcohols to produce carbocations. Carbocations are key intermediates in the transformations of hydrocarbons.
- Inorganic compounds are sometimes synthesized in strongly acidic or basic media. For example, ternary metal oxides can be synthesized and crystallized in molten NaOH or KOH, which are strongly basic. Organic fluorination reactions are often done in strongly acidic media, such as anhydrous HF. Understanding the familiar chemistry of acids and bases in water helps us understand how these non-aqueous media work.
- The acidic or basic environment of metal ions affects the stability of their oxidation states. We will learn more about this in Chapter 4.
- Transition metal complexes (coordination compounds and organometallic compounds) are essentially Lewis acid-base complexes. We can understand a great deal about their stability and reactivity by considering the acid-base character of metals and ligands. We will learn about this in Chapter 5.
Learning goals for Chapter 3:
- Understand the Bronsted and Lewis definitions of acids and bases.
- Identify conjugate acids and bases, and rules for strong & weak acids/bases, in both Bronsted and Lewis acid-base systems.
- Use Pauling’s rules to predict the pKas of oxoacids.
- Understand the periodic trends of acidic, basic, and amphoteric compounds
- Predict, describe, and rationalize acid/base chemistry in non-aqueous systems, including acidic and basic solvents, aprotic solvents, and molten salts.
- Apply the principles of acid-base chemistry to the design of molecules and Lewis acids with target functions.
- Understand the connection between acid-base chemistry and the stabilization of oxidation states.
- Predict favorable and stable compounds using hard-soft acid-base (HSAB) theory.
- Understand the applications of the ECW model.
3.1 Brønsted and Lewis acids and bases
[edit | edit source]
Three theories of acids and bases. There are three major classifications of substances known as acids or bases. The Arrhenius definition states that an acid produces H+ in solution and a base produces OH-. This theory was developed by Svante Arrhenius in 1883. Later, two more sophisticated and general theories were proposed. These are the Brønsted-Lowry and the Lewis definitions of acids and bases. The relationship between these theories is illustrated in the figure at the left.
In acid-base chemistry, the hydrogen ion is often called and written as a proton (H+), but in fact it exists in a variety of hydrated forms that include H3O+, H5O2+, H7O3+. When we write H+(aq) or H3O+(aq) we are referring to the dynamic equilibrium mixtures of these forms of protonated water.
The Arrhenius theory, which is the simplest and least general description of acids and bases, includes acids such as HClO4 and bases such as NaOH or Mg(OH)2. This theory successfully describes how acids and bases react with each other to make water and salts. However, it does not explain why some substances that do not contain hydroxide ions, for example F- and NO2-, can make basic solutions in water. The Brønsted-Lowry definition of acids and bases addresses this problem. In this theory an acid is a substance that can release a proton (like in the Arrhenius theory) and a base is a substance that can accept a proton. A basic salt such as Na+F- generates OH- ions in water by taking protons from water itself (to make HF):
- F-(aq) + H2O(l) ⇌ HF(aq) + OH-
When a Brønsted acid dissociates, it increases the concentration of hydrogen ions in the solution, [H+]; conversely, Brønsted bases dissociate by taking a proton from the solvent (water) to generate [OH-].
- Acid dissociation: HA(aq) ⇌ A-(aq) + H+(aq)
- Ka=[A-][H+]/[HA]
- Base dissociation: B(aq) + H2O(l) ⇌ HB+(aq) + OH-(aq)
- Kb = [HB+][OH-]/[B]
Conjugate acids and bases. One important consequence of these equilibria is that every acid (HA) has a conjugate base (A-), and vice-versa. In the base dissociation equilibrium above the conjugate acid of base B is HB+.
For a given acid or base, these equilibria are linked by the water dissociation equilibrium:
- H2O(l) ⇌ H+(aq) + OH−(aq)
- Kw = [H+][OH−]
for which the equilibrium constant Kw is 1.00 × 10−14 at 25°C. It can be easily shown that the product of the acid and base dissociation constants Ka and Kb is Kw.
Strong and weak acids and bases. Acids and bases that dissociate completely are said to be strong:
- HClO4(aq) → H+(aq) + ClO4−(aq)
- HBr(aq) → H+(aq) + Br−(aq)
- CH3O−(aq) + H2O(l) → CH3OH(aq) + OH−(aq)
- NH2−(aq) + H2O(l) → NH3(aq) + OH−(aq)
Here the right-handed arrow (→) implies that the reaction goes to completion. That is, a 1.0 M solution of HClO4 in water actually contains 1.0 M H+(aq) and 1.0 M ClO4−(aq), and very little undissociated HClO4.
Conversely, weak acids such as acetic acid (CH3COOH) and weak bases such as ammonia (NH3) dissociate only slightly in water - typically a few percent, depending on their concentration and the values of Ka and Kb - and exist mostly as the undissociated molecules.

Example: Household ammonia is a solution of NH3 in water that ranges from about 5-10% by weight. Let’s calculate the percent ionization and the pH of the solution.
For a solution that is 8% ammonia by weight, assuming that the density is about the same as that of liquid water, the analytical concentration of ammonia is (80 g/L) / (17 g/mol) = 4.7 M.
The other thing we need to know to solve this problem is the base dissociation constant, Kb.
- NH3 + H2O ⇌ NH4+ + OH− Kb = 1.8 × 10−5
We can solve this problem rigorously by invoking both charge balance ([H+] + [NH4+] = [OH−]) and mass balance (4.7 M = [NH3] + [NH4+]) and using Kw = [H+][OH−]. But because the algebra becomes complicated with that method - leading to a cubic equation that is hard to solve - we’ll invoke two simplifying assumptions:
- [NH4+] ≈ [OH−] >> [H+] (which is a reasonable assumption for a basic solution)
- and
- [NH3] >> [NH4+] (also reasonable if the percent ionization is small)
Now we can write:
- [NH4+][OH−] ≈ [OH−]2 = (4.7 M)(Kb) = 8.4 × 10−5
- [OH−] = 9.2 × 10−3 M (≈ [NH4+]), [H+] = Kw/[OH−]= 1.1 × 10−12 M, pH = 11.97
The percent ionization is:
- 100% x 9.2 x 10−3 M / 4.7 M = 0.19%
This example illustrates that it is technically incorrect to label a bottle of aqueous ammonia as “ammonium hydroxide,” since only about 2/10 of one percent of the weak base exists in that form.
Conjugate acids and bases. A common misconception is that strong acids have weak conjugate bases, and that weak acids have strong conjugate bases. It is easy to see that this is incorrect by remembering that KaKb = Kw. Our definition of a strong acid or base is that K >> 1, i.e., that the substance dissociates completely. Our definition of a weak acid or base is 1 > K > Kw. It follows that if Ka >> 1 (strong) then Kb cannot be > Kw (weak).
In fact, strong acids such as HCl dissociate to produce spectator ions such as Cl− as conjugate bases, whereas weak acids produce weak conjugate bases. This is illustrated below for acetic acid and its conjugate base, the acetate anion. Acetic acid is a weak acid (Ka = 1.8 x 10−5) and acetate is a weak base (Kb = Kw/Ka = 5.6 x 10−10)
![]()
The strength of a conjugate acid/base varies inversely with the strength or weakness of its parent acid or base. Any acid or base is technically a conjugate acid or conjugate base also; these terms are simply used to identify species in solution (i.e acetic acid is the conjugate acid of the acetate anion, a base, while acetate is the conjugate base of acetic acid, an acid).
Neutral oxyacids (H2SO4, H3PO4, HNO3, HClO2, etc.) can be classified as strong or weak following a simple rule first noted by Linus Pauling. If the number of oxygen atoms exceeds the number of hydrogen atoms by two or more, then the acid is strong; otherwise it is weak. For example HClO4 and HClO3, where the difference is 3 and 2, respectively, are both strong acids. HNO2 and HClO2 are both weak because the difference is 1 in both cases. For weak acids, the relative strength depends on this difference (i.e., HClO2 is a stronger weak acid than HOCl) and on the electronegativity of the central atom (HOCl is stronger than HOI).
Acids that can donate more than one proton are called polyprotic acids. For example, sulfuric acid, H2SO4, is a strong acid that has a conjugate base that actually happens to be a weak acid itself. This means that every mole of H2SO4 in aqueous solution donates more than 1 mole of protons. Carbonic acid (H2CO3) and phosphoric acid (H3PO4) are weak polyprotic acids. Typically, the sequential pKa's of polyprotic acid are separated by about 5 pH units, because it becomes progressively more difficult to remove protons as the ion becomes more negatively charged. For example, the three pKa's of phosphoric acid are 2.15, 7.20, and 12.35.

Amphoteric compounds. Some substances can act either as an acid and as a base. An example is water. H2O molecules may either donate a hydrogen ion or accept one. This property makes water an amphoteric solvent. In the situation where an acid dissociates in solution, water is acting as a base. Conversely, water acts as an acid when bases dissociate. The strongest acid we can make in H2O is H+ (aq), and the strongest base we can make in H2O is OH− (aq).
Other examples of amphoteric compounds are oxides and hydroxides of elements that lie on the border between the metallic and non-metallic elements in the periodic table. For example, aluminum hydroxide (Al(OH)3) is insoluble at neutral pH, but can accept protons in acid to make [Al(H2O)6]3+ or accept an OH− ion in base to form Al(OH)4− ions. Consequently, aluminum oxide is soluble in acid and in base, but not neutral water. Other examples of amphoteric oxides are BeO, ZnO, Ga2O3, Sb2O3, and PbO. Increasing the oxidation state of a metal increases the acidity of its oxide by withdrawing electron density from the oxygen atoms. Thus, Sb2O5 is acidic, but Sb2O3 is amphoteric.
Solvent leveling. Solvent leveling is an effect that occurs when a strong acid is placed in a solvent such as (but not limited to) H2O. Because strong acids donate their protons to the solvent, the strongest possible acid that can exist is the conjugate acid of the solvent. In aqueous solution, this is H3O+. This means that the strength of acids such as HCl and HBr cannot be differentiated in water as they both are dissociated 100% to H3O+. In the context of our discussion of conjugate bases above, we would say that both Cl− and Br− are spectator ions in water: neither one is a strong enough base to accept a proton from H3O+. In order to differentiate the acidities of strong acids such as HClO4 and HCl, or the basicities of strong bases such as CH3O− and NH2−, we must typically work in non-aqueous solvents, as explained below.
Nonaqueous solutions. The Brønsted theory encompasses any type of solvent that can donate and accept H+ ions, not just aqueous solutions. The strength of an acid or a base varies depending on the solvent. Non-aqueous acid-base chemistry follows similar rules to those developed for acids and bases in water. For example in liquid ammonia, the solvent autodissociates in the reaction:
- 2NH3(l) ⇌ NH4+ + NH2−
This equilibrium is analogous to the autodissociation of water, but has a smaller equilibrium constant (K ≈ 10−30). It follows by analogy to water that NH4+ is the strongest acid and NH2− is the strongest base that can exist in liquid ammonia. Because ammonia is a basic solvent, it enhances the acidity and suppresses the basicity of substances dissolved in it. For example, the ammonium ion (NH4+) is a weak acid in water (Ka = 6 x 10−10), but it is a strong acid in ammonia. Similarly, acetic acid is weak in water but strong in ammonia. Solvent leveling in fact makes HCl, CH3COOH, and NH4Cl all strong acids in ammonia, where they have equivalent acid strength.
Strong acids that are leveled in water have different acid strengths in acidic solvents such as HF or anhydrous acetic acid. For example, acid dissociation of HX in acetic acid (CH3COOH) involves protonating the solvent to make its conjugate acid (CH3COOH2+) and the X− anion. Because CH3COOH2+ is a stronger acid than H3O+, the anion X− (which is a spectator in water) can become a weak base in CH3COOH:
- HX + CH3COOH ⇌ CH3COOH2+ + X−
It follows that acidic solvents magnify the Brønsted basicities of substances that cannot accept protons in water. Conversely, basic solvents magnify the acidity of substances that cannot donate a proton to OH−.
The acidity and basicity of non-aqueous solvents is difficult to quantify precisely, but one good relative measure is the Hammett acidity function, Ho. Ho is defined analogously to pH according to the Henderson-Hasselbach equation:
Ho = pKa + log([base]/[conjugate acid])
For non-aqueous solvents, or for acidic or basic compounds in dissolved in solvents that do not themselves dissociate, Ho is a rough measure of the pH of the solvent or compound in question. Anhydrous HF and H2SO4 have Ho values of approximately -10 and -12 respectively.
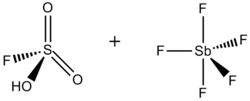
Superacids and superbases. The term superacid was originally coined by James Bryant Conant in 1927 to describe acids that were stronger than conventional mineral acids.[1] George A. Olah prepared the so-called magic acid, so-named for its ability to attack hydrocarbons, by mixing antimony pentafluoride (SbF5) and fluorosulfonic acid (FSO3H). The name was coined after a candle was placed in a sample of magic acid. The candle dissolved, showing the ability of the acid to protonate hydrocarbons, which under aqueous acidic conditions cannot be protonated.
At 140 °C , FSO3H–SbF5 converts methane into the tertiary-butyl carbocation, a reaction that begins with the protonation of methane:[2]
- CH4 + H+ → CH+5
- CH+5 → CH+3 + H2
- CH+3 + 3 CH4 → (CH3)3C+ + 3H2
Fluoroantimonic acid, HSbF6, can produce solutions with H0 down to –28.[3] Fluoroantimonic acid is made by combining HF and SbF5. In this system, HF releases its proton (H+) concomitant with the binding of F− by antimony pentafluoride, which (as described below) is a Lewis acid. The resulting anion (SbF6-) is both a weak nucleophile and an extraordinarily weak base.
Superacids are useful in reactions such as the isomerization of alkanes. Industrially, anhydrous acid-exchanged zeolites, which are superacid catalysts, are used on a massive scale to isomerize hydrocarbons in the processing of crude oil to gasoline. Superbases such as lithium diethylamide (LiNEt2), alkyllithium compounds (RLi), and Grignard reagents (RMgX) useful in a broad range of organic reactions. LiNEt2 deprotonates C-H bonds to generate reactive carbanions. RLi and RMgX are powerful nucleophiles.
The use of superbases in nonaqueous media allows us to rank the acidities (and measure the pKa's) of different classes of molecules. This ranking is particularly important in understanding the reactions of organic molecules. Note that the order of acidities for hydrocarbons is alkynes >> alkenes, aromatics >> alkanes. This ordering has to do with the hybridization of the carbon atom that forms the carbanion. The negatively charged lone pair of the carbanion is stabilized in orbitals that have high s character (e.g., sp vs. sp2 or sp3). This is because s orbitals have finite probability density at the nucleus and "feel" the positive nuclear charge (thereby stabilizing the extra negative charge on carbon) more than p orbitals. Resonance effects also stabilize carbanions. Thus, cyclopentadiene is more acidic than even an alkyne because the negative charge is delocalized over the entire (aromatic) C5H5- ring when the C5H6 is deprotonated.
| name | formula | structural formula | pKa |
|---|---|---|---|
| Methane | CH4 | 
|
56 |
| Propene | C3H6 | 44 | |
| Benzene | C6H6 | 43 | |
| Acetylene | C2H2 | 
|
25 |
| Cyclopentadiene | C5H6 | 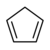
|
18 |
| Table 1. Carbon acid acidities in pKa in DMSO [4]. | |||
Acid-base equilibria in molten salts. When a solid salt melts, it forms a solution of the cations and anions. For example, KOH melts at temperatures above 400 °C and dissociates into K+ and OH- ions which can act as a solvent for chemical reactions. Because of the autodissociation of the OH- solvent, water is always present in a molten KOH flux, according to the acid-base equilibrium:
- 2 OH- ⇌ H2O + O2-
It follows that in this very basic solvent, water (the conjugate acid of the solvent) is the strongest acid that can exist. The conjugate base of the solvent, O2-, is the strongest base. This autodissociation equilibrium allows for the acidity of a flux to be easily tuned through the addition or boiling off of water. A "wet" flux is more acidic, and can dissolve metal oxides that contain the basic O2- anion. Conversely a "dry" flux is more basic and will cause oxides to precipitate. Molten hydroxide fluxes can thus be used in the synthesis of oxide crystals, such as the perovskite superconductor (K1-XBaXBiO3).[5]. Eutectic mixtures of NaOH and KOH are relatively low melting (≈ 200 °C) and can be used as solvents for crystallizing a variety of basic oxides.
Lewis Acids and Bases
The Lewis classification of acids and bases is broader than the Brønsted-Lowry definition, and encompasses many more substances. Whereas the Brønsted-Lowry and the Arrhenius classifications are based on transfer of protons, Lewis acidity and basicity are based on the sharing of an electron pair. Lewis acids can accept an electron pair, while Lewis bases can donate an electron pair. This definition encompasses the Brønsted-Lowry definition, in that H+ is an electron pair acceptor (when interacting with a base), and a base is an electron pair donor in its interaction with H+. This is illustrated below for the protonation of ammonia.

Boron trifluoride, BF3 acts as a Lewis acid when it combines with a basic ion or molecule that can donate an electron pair. Such a reaction is shown below.
- BF3 + F- ⇌ BF4-
Here, the acid is BF3 and the base is F-. This acid-base reaction allows boron (which is electron-deficient in BF3) to complete its octet. Similarly, AlCl3 is a Lewis acid that can react with Cl- (a Lewis base) to make the Lewis "salt" AlCl4-. Note that in water Cl- is a spectator ion (a weaker base than the solvent) in Brønsted acid-base reactions.
Additional examples of Lewis acid base reactions. In each, try to identify the acid, the base, and the salt, based on the concept that the base is the molecule or ion that donates an electron pair. In cases where you are not sure, it may help to draw the VSEPR structures of the molecules:
- I2 + I- ⇌ I3-
- AuCl3 + Cl- ⇌ [AuCl4]-
- Fe3+ + 6 H2O ⇌ [Fe(H2O)6]3+
- TiF4 + 2 F- ⇌ [TiF6]2-
- SF4 + SbF5 ⇌ "SSbF9"
In other Lewis acid base reactions both acid and base are molecules and the product is referred to as an adduct.
- (CH3)3 B + N(CH3)3 →(CH3)3 B-N (CH3)3
- I2 + S(CH3)2 → I2-S(CH3)2
- C5N5N + Cu(HFacac)2 → C5N5N-Cu(HFacac)2
Lewis acidity is the basis for coordination chemistry, a topic we will discuss in more detail in Chapter 5. This is because coordination chemistry involves metal ions that are Lewis acids, which bond to ligands that are Lewis bases.
Determining the strength of metal ion Lewis acids. There are three determining factors in the Lewis acid strength of a metal ion:
1. The higher positive charge on the metal, the more acidic it is. For example, Al3+ and Fe3+ are good Lewis acids and their salts make acidic solutions in water, but K+ and Na+ are not.
2. The smaller the atomic radius of the metal ion, the more acidic it is. Going down the periodic table, the Lewis acidity of metal ions decreases (e.g., Al3+ > Ga3+ > In3+) because the ionic radius increases.
3. For transition metal ions, more electronegative metals tend to make stronger Lewis acids. The electronegativity has maxima at W and Au in the 5d series, so metal ions near in that part of the periodic table are good Lewis acids.
Molecules with five coordinate geometries (e.g., PCl5, AsF5, SbF5) are typically strong Lewis acids, because when accepting another pair of electrons from a base, they form an octahedral molecule or anion. Neither of the common five-coordinate geometries (trigonal bipyramidal or square pyramidal) is efficient in terms of packing. The Lewis acid-base reaction forms an additional bond with a relatively small energetic penalty of stretching the existing bonds:

Because F- is a good Lewis base, and also a small anion, it can form stable octahedral anions with both main group elements and transition metals. For this reason, TiO2 and SiO2 dissolve in HF (but are unreactive with aqueous HCl and other strong acids):
- TiO2 + 4 HF + 2 F- ⇌ TiF62- + 2 H2O
- SiO2 + 4 HF + 2 F- ⇌ SiF62- + 2 H2O

Lewis bases stabilize high oxidation states. An interesting example of using Lewis acid-base chemistry to drive reactions is the chemical synthesis of fluorine gas, which was devised by Karl O. Christe in 1986.[6] Christe at the time was organizing a symposium to commemorate the 100th anniversary of the isolation of elemental fluorine by Henri Moissan, which Moissan did in 1886 by electrolyzing a solution of anhydrous HF. 100 years later, there was still no direct (non-electrochemical) synthesis of F2. Christe's reaction scheme followed two steps. The first was the known synthesis of K2MnF6 from KMnO4:
- 4 MnO4-(aq) + 10 H2O(l) + 24 F-(aq) → 4 MnF62-(aq) + 3O2(g) + 20 OH-(aq)
- 2 K+(aq) + MnF62-(aq) → K2MnF6(s)
The second step involved reacting K2MnF6 with the powerful Lewis acid SbF5, to make metastable MnF4, which decomposes spontaneously to MnF3 and fluorine gas:
- K2MnF6(s) + 2 SbF5(l) → 2 KSbF6(s)+ "MnF4"(s)
- "MnF4"(s) → MnF3(s) + 1/2 F2(g)
This reaction teaches us something interesting and important about the connection between acid-base and redox chemistry. Acids tend to stabilize low oxidation states, and bases stabilize high oxidation states (We will see this again soon in Chapter 4, in the context of Pourbaix diagrams). Mn is stable in the +4 oxidation state in K2MnF6, where it is surrounded by six basic F- anions. However, the highest stable neutral fluoride of Mn is MnF3, and MnF4 (transiently formed from K2MnF6) spontaneously decomposes to generate fluorine.
Oxide is a better base than fluoride. Interestingly, Mn can lose all its valence electrons to form Mn7+ in the permanganate ion, MnO4-. Here the 7+ oxidation state is stabilized electrostatically by coordination to four O2- ions, and by the overall -1 charge on the MnO4- anion. Because of its 2- charge, O2- is a stronger base and a better ion for stabilizing high oxidation states than F-. This is a general trend among transition metals: the highest oxidation state is usually reached in the oxide, not in the fluoride, despite the fact that F is a more electronegative element than O. For example, Cr6+ is stable in the CrO42- and Cr2O72- anions, but not in any neutral fluoride or fluoroanion. The +8 oxidation state occurs in RuO4 and OsO4, but not in any fluoride of Ru or Os.
3.2 Hard and soft acids and bases
[edit | edit source]Lewis acids and bases can be classified by designating them as hard or soft.
- Hard Acids/Bases:
- "Hard" acids and bases have a high charge (positive for acids, negative for bases) to ionic radius ratio along with higher oxidation states. Hard acids are not very polarizable and have high charge densities. Thus metal ions with high positive charges and smaller ionic sizes tend to be hard acids. Early transition metal ions in the 3d series tend to be hard Lewis acids. Hard bases are typically small anions and neutral molecules. Some examples of hard acids and bases include: H+, O2-, OH-, F-, Fe3+, and Al3+.
- Soft Acids/Bases:
- "Soft" acids or bases have a low charge to radius ratio, with low oxidation states. They are normally larger ions that are polarizable. For example, I- and S2- are soft bases and low charge density transition metals, such as Ag+, are considered soft acids. Soft acids often include transition metals in the second and third row of the periodic table that have a +1 or +2 charge, as well as late transition metals (especially those in the 4d and 5d series) with filled or almost completely filled d orbitals.
Acids and bases are not strictly hard or soft, with many ions and compounds being classified as intermediate. For example, trimethylborane, Fe2+, and Pb2+ cations are intermediate acids, and pyridine and aniline are examples of intermediate bases. An element can also change its hard/soft character depending on its oxidation state. The most extreme example is hydrogen, where H+ is a hard acid and H- is a soft base. Ni3+ (as in the layered compound NiOOH) is a hard acid, but Ni0 (as in Ni(CO)4) is a soft acid. The figures below show hard/soft trends for acids (left) and bases (right) in the periodic table. For bases, the major hard/soft discontinuity is between the 2nd row (N, O, F) and the rows below.


Like binds with Like
Hard acids interact more strongly with hard bases than they do with soft bases, and soft acids interact more strongly with soft bases than hard bases. Thus, the most stable complexes are those with hard-hard and soft-soft interactions. This tendency is illustrated in the table below, which shows the trend in formation constants for hard and soft acids. Hard acids bind halides in the order F- > Cl- > Br- > I-, whereas soft acids follow the opposite trend.
Log K1 |
fluoride |
chloride |
bromide |
iodide |
acid
classification
|
|---|---|---|---|---|---|
Fe3+ |
6.0 |
1.4 |
0.5 |
- |
Hard
|
Pb2+ |
1.3 |
0.9 |
1.1 |
1.3 |
Intermediate
|
Ag+ |
0.4 |
3.3 |
4.7 |
6.6 |
Soft
|
Hg2+ |
1.0 |
6.7 |
8.9 |
12.9 |
Soft
|

The softest metal ion in the periodic table is Au+(aq). It forms stable complexes with soft bases such as phosphines and CN-, but not with hard bases such as O2- or F-. The affinity of Au+ for the soft base CN- is high, and the resulting [Au(CN)2]- complex is so stable that gold (which is normally very difficult to oxidize) can be oxidized by oxygen in the air:
- 4 Au(s) + 8 CN-(aq) + O2(g) + 2 H2O ⇌ 4 [Au(CN)2]-(aq) + 4 OH-
This reaction is used in gold mining to separate small flakes of Au from large volumes of sand and other oxides. Ag is similarly dissolved by air oxidation in cyanide solutions. The precious metals are then isolated from the solution using chemical reducing agents or by electroplating. The use of cyanide ion on a large scale in mining, however, creates a potentially serious environmental hazard. In 2000, a spill at Baia Mare, Romania resulted in the worst environmental disaster in Europe since Chernobyl. Cyanide, which is highly toxic, is gradually oxidized by air to the less toxic cyanate (OCN-) ion. On the laboratory scale, cyanide plating solutions are typically disposed of by using bleach to oxidize CN- to OCN-, and the metal is recovered as an insoluble chloride salt.
The Au3+ ion, because of its higher positive charge, is a harder acid than Au+ and can form complexes with harder bases such as H2O and amines. In keeping with the "like binds like" principle, the compound AuI (soft-soft) is stable, but AuI3 (hard-soft) is unknown. Conversely, AuF has never been isolated but AuF3 (hard-hard) is stable.
3.3 The electrostatic-covalent (ECW) model for acid-base reactions
[edit | edit source]The classification of Lewis acids and bases as hard and soft is a useful qualitative approach to rationalize their behavior. The ECW Model is a more quantitative model that describes and predicts the strength of Lewis acid – Lewis base interactions. The strength of the acid-base interaction is measured as the enthalpy of adduct formation, △H . Each acid is characterized by an EA and a CA. Each base is likewise characterized by its own EB and CB. The E and C parameters refer, respectively, to the electrostatic and covalent contributions to the strength of the bonds that the acid and base will form. These parameters have been empirically obtained by using enthalpies for adducts that form only σ bonds between the acid and base as well as adducts that have no steric repulsion between the acid and base.
-△H = EAEB + CACB + W
This equation reproduces and predicts the enthalpy change, △H, of a reaction between many acids and a bases. △H is a measure of strength of the bond between the acid and the base, both in the gas phase and in weakly solvating media. The W term represents a constant energy for cleavage of a dimeric acid or base. For example, the enthalpy of cleavage of [Rh(CO)2Cl]2 by base B involves two steps. The first step is cleavage of the dimer, which is W:
½ [Rh(CO)2Cl]2 → Rh(CO)2Cl W = 43.5 kJ/mol
The second step is the binding of B to the RhCl(CO)2 monomer. In another case, W is the enthalpy needed to cleave the internal hydrogen bonding of the H-bonding acid (CF3)3COH.
The calculation of the enthalpy of adduct formation for the reaction of pyridine, C5H5N and bis(hexafloroacetyacetonato)copper (II), Cu(HFacac)2, shows how these parameters are used. In this case W =0 since neither the acid nor the base is a dimer. Selected parameters can be found at the Wikipedia page for the ECW Model
-△H = EAEB + CACB = (1.82)(1.78) + ( 2.86)(3.54) = 13.4 kcal/mol = -56.1 kJ/mol
△H = -56.1 kJ/mol, △Hmeasured = -56.1 kJ/mol.
However, the ᐃH calculated for the reaction of Me3B with Me3N is more negative than that observed. This discrepancy is attributed to steric repulsion between the methyl groups on the B and N atoms. The difference between the calculated and observed values can then be taken as the amount of the steric effect, a value otherwise not attainable. Steric effects have also been identified with (CH3)3SnCl and with Cu(HFacac)2. When π bonding contributes to the measured enthalpy, the enthalpy calculated from the E and C parameters will be less than the measured enthalpy and the difference provides a measure of the extent of the π bonding contribution.
A graphical presentation of this model clearly shows that there is no single ranking order of Lewis acid or Lewis base strengths, a point often overlooked, and emphasizes that the magnitude of acid and base interactions requires two parameters (E & C) to account for the interactions. A Cramer-Bopp plot[7]using the three Lewis bases: acetonitrile, ammonia, and dimethyl sulfide illustrates that there is no unique ordering of Lewis base strengths. The Cramer-Bopp plot is a visual tool for comparing Lewis base strengths with the range of possible Lewis acid partners, and similar plots can be constructed to examine selected Lewis acids against the range of possible Lewis bases. These plots show that two properties are needed to completely define acid and base strength and that any attempt to define strength with one property or parameter is limited in its utility. For Drago’s quantitative ECW model the two properties are electrostatic and covalent while for Pearson's qualitative HSAB theory the two properties are hardness and strength.
3.4 Frustrated Lewis pairs
[edit | edit source]A frustrated Lewis pair (FLP) is a compound or mixture that contains a Lewis acid and a Lewis base which, because of steric hindrance, cannot combine to form a classical adduct.[8] Many kinds of FLPs have been devised, and their reactivity towards other molecules has been broadly developed.[9][10]
The hydrogen adduct of the original FLP, a phosphonium-borate salt, can be prepared by combining a phenylene bridged phosphinoborane and dihydrogen. The salt, which is colorless, is stable in the presence of air and moisture. It releases molecular H2 when heated above 100 °C. This reactivity is remarkable considering the strength of the H-H bond, 432 kJ/mol.
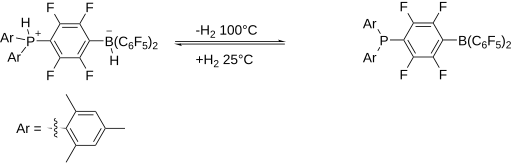
The discovery that some FLPs can split H2[11] triggered the rapid growth of research into FLP's. Because of their "unquenched" reactivity, such systems are reactive toward substrates that can undergo heterolysis. For example, many FLP's split the hydrogen molecule. This reactivity suggested that FLP's can be useful for hydrogenation reactions. A sizable range of homogeneous and heterogeneous catalytic reactions have now been developed using FLP's.
Mixtures of sterically hindered Lewis acids and bases also can act as FLPs. One successful strategy is to mix sterically hindered triarylphosphines with triarylboranes. Small molecules such as CO2 and ethylene can then form a bridge between the phosphine Lewis base and borane Lewis acid, e.g.,
- P(t-Bu)3 + B(C6F5)3 + CO2 → (t-Bu)3P+C(O)OB−(C6F5)3
- PCy3 + B(C6F5)3 + C2H4 → Cy3P+CH2CH2B−(C6F5)3
Because FLPs behave at the same time as both nucleophiles and electrophiles, they can effect the ring-opening of cyclic ethers such as THF, 2,5-dihydrofuran, coumaran, and dioxane.[12]
3.5 Discussion questions
[edit | edit source]- Discuss periodic trends in the Lewis acidity of metal ions.
- Explain what we mean by hard and soft acids and bases, using specific examples.
- Explain why hard and soft should not be equated with electrostatic and covalent.
- According to HSAB theory Cu and Zn are classified as intermediate acids while Cd is classified as soft. The base (CH2)4O is considered hard while (CH2)4S is soft. Using E and C numbers for Cd[N(Si(CH3)3]2 and ZnTPP calculate the enthalpies for these two acids interacting with (CH2)4O and (CH2)4S and show that the HSAB model correctly predicts which base interacts more strongly with which acid. Do the same comparison using Cu(HFacac)2 as the acid and show that HSAB fails to predict which base interacts more strongly. How are these results related to the Cramer-Bopp plot that show one property or one parameter cannot be used to rank Lewis acid or base strength?
3.6 Problems
[edit | edit source]1. Classify each of the substances below as an acid or base, and as strong or weak or as a spectator in the solvent listed. For each case, write out the dissociation reaction.
(a) H3PO4 in water
(b) HBr in glacial acetic acid
(c) CH3CH2COOH in liquid ammonia
(d) H2O in molten KOH
(e) I- in liquid ammonia
2. Write out the dissociation reactions for an acid HA and its conjugate base A- in water. Use the equilibrium expressions for Ka and Kb to prove that KaKb = Kw.
3. Calculate the percent ionization of 1 M acetic acid (Ka = 1.8 x 10-5) in water.
4. For each pair of compounds below, indicate which one is a stronger acid and explain your choice:
(a) [Co(H2O)6]3+ or [Co(H2O)6]2+
(b) H3BO3 or H3AsO3
(c) [In(H2O)6]3+ or [Ga(H2O)6]3+
(d) HClO3 or HClO4
(e) H3PO4 or HMnO4
5. The Al3+ and Co3+ cations have similar ionic radii, but the acidities of aqueous nitrate solutions containing the two ions are different. Explain which ion is more acidic and why.
6. Fluorine is more electronegative than oxygen, but the highest oxidation states of transition metals are typically found in the oxides. For example, manganese metal reacts with fluorine to make MnF3, but higher oxidation states of Mn do not exist among its binary fluorides. However, Mn(IV) is stable in oxides such as MnO2 and CaMnO3, and also in fluoride salts such as K2MnF6. Explain why these compounds can stabilize a higher oxidation state of manganese than binary fluorides.
7. (a) When solutions of the hydrosulfide (SH-) salts of As3+, Sb3+, and Sn4+ are reacted with aqueous solutions of ammonium hydrosulfide (NH4+SH-), the sulfide salts As2S3, Sb2S3, and SnS2 precipitate. When excess aqueous Na2S is added, however, these sulfides re-dissolve to form soluble anionic complexes. In contrast, solutions of Cu2+, Pb2+, Hg2+, Bi3+ and Cd2+ precipitate as solid sulfides but do not re-dissolve in solutions that contain excess sulfide. We may consider the first group of ions to be amphoteric for soft acid-base reactions that involve SH- instead of OH-. The second group is more basic (less acidic) in the sense that it does not react with excess soft base. Use this information to locate the amphoteric diagonal in the periodic table for sulfides. Compare this with the diagonal that defines the amphoteric oxides, which dissolve in either acidic or basic solutions. Does your analysis agree with the description of S2- as a softer base than O2-?
(b) Au+ is also precipitated by SH- ions, and the sulfide Au2S redissolves to form a soluble complex in excess SH-. Does this fit the trend you discovered in part (a)? Does it make sense in terms of the electronegativity of Au? Explain.
8. Calculate the enthalpy for the adduct (CH3)3B-N(CH3)3 and compare it to the measured value of -73.6 kJ/mol. Parameters can be found at ECW Model.
9. Calculate the enthalpy for the adduct formation for the Lewis acid (CH3)3SnCl with each of the following Lewis bases: (C2H5)2O, (CH2)4O, and CH3CN. Their respective measured enthalpies are -9.2, -21.3 and -20.1 kJ/mol. Explain the any differences between the calculated and measured enthalpies. Parameters can be found at ECW Model.
10. Refer to the Cramer-Bopp plot found at ECW Model and indicate the order of increasing Lewis base strength for the following:
(a) An acid with Ra = -0.5
(b) An acid with Ra = 0.33
(c) An acid with Ra = -0.9
11. The Lewis acid [Rh(CO)2Cl]2 has a W value of -43.47 kJ/mol. The E, C, and W parameters found at ECW Model give the enthalpy for forming one mole adduct with a base B, that is, B + 1/2 [Rh(CO)2Cl]2 → B-RhCl(CO)2
(a) What does W refer to?
(b) What is the heat of dissociation of one mole of [Rh(CO)2Cl]2 ?
(c) What is the enthalpy of 2B + [Rh(CO)2Cl]2 → 2 B-RhCl(CO)2 ?
(d) Calculate the enthalpy per mol of B-RhCl(CO)2 when B = C5H5N, and when B = (CH3)2CO.
12. When aluminum corrodes, it forms solid Al2O3 on its surface, but when silver tarnishes it forms solid Ag2S on its surface. Explain why this is so.
13. Given the reactions below, explain if the reaction favors reactants or products at equilibrium. Briefly explain your reasoning.
[Fe(CO)6]Cl3 (aq) + 6 NH3(g) ⇌ [Fe(NH3)6]Cl3(aq) + 6 CO(g)
[Pd(CN)4]2-(aq) + [Mn(CO3)4]2-(aq) ⇌ [Mn(CN)4]2+(aq) + [Pd(CO3)4]6-(aq)
14. Rationalize the differences in formation constants for each pair of reactions.
(a) Iron
Fe2+(aq) + 4 OH−(aq) → [Fe(OH)4]2−(aq). Kf = 1.00 × 1010
Fe3+(aq) + 4 OH−(aq) → [Fe(OH)4]−(aq). Kf = 2.51 × 1034
(b) Copper
Cu+(aq) + 4 CN−(aq) → [Cu(CN)4]3−(aq). Kf = 2.00 × 1030
Cu2+(aq) + 4 CN−(aq) → [Cu(CN)4]2−(aq). Kf = 1.00 × 1025
15. Describe the differences in solubility for the following pairs. How could you predict these solubility patterns?
(a) AgF(s) is water soluble, while AgI(s) is not soluble in water.
(b) LiF(s) is insoluble in water, while LiI(s) is soluble.
3.7 References
[edit | edit source]- ↑ Hall NF, Conant JB (1927). "A Study of Superacid Solutions". Journal of the American Chemical Society. 49 (12): 3062–70. doi:10.1021/ja01411a010.
- ↑ George A. Olah, Schlosberg RH (1968). "Chemistry in Super Acids. I. Hydrogen Exchange and Polycondensation of Methane and Alkanes in FSO3H–SbF5 ("Magic Acid") Solution. Protonation of Alkanes and the Intermediacy of CH5+ and Related Hydrocarbon Ions. The High Chemical Reactivity of "Paraffins" in Ionic Solution Reactions". Journal of the American Chemical Society. 90 (10): 2726–7. doi:10.1021/ja01012a066.
- ↑ Herlem, Michel (1977). "Are reactions in superacid media due to protons or to powerful oxidising species such as SO3 or SbF5?". Pure & Applied Chemistry. 49: 107–113. doi:10.1351/pac197749010107.
- ↑ Equilibrium acidities in dimethyl sulfoxide solution Frederick G. Bordwell Acc. Chem. Res.; 1988; 21(12) pp 456 - 463; DOI:10.1021/ar00156a004
- ↑ R. J. Cava; et al. (1988). "Superconductivity near 30 K without copper: the Ba0.6K0.4BiO3 perovskite". Nature. 332: 814–6. doi:10.1038/332814a0.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ↑ Christe, Karl O. (1986). "Chemical synthesis of elemental fluorine". Inorganic Chemistry 25 (21): 3721. doi:10.1021/ic00241a001.
- ↑ Cramer RE, Bopp TT (1977). "Great E and C plot. Graphical display of the enthalpies of adduct formation for Lewis acids and bases". Journal of Chemical Education. 54 (10): 612–613. doi:10.1021/ed054p612.
- ↑ Stephan, D. W. (2008). "Frustrated Lewis pairs: a concept for new reactivity and catalysis". Org. Biomol. Chem. 6: 1535–1539. doi:10.1039/b802575b.
- ↑ Stephan, D. W.; Erker, G. (2010). "Frustrated Lewis Pairs: Metal-free Hydrogen Activation and More". Angewandte Chemie International Edition. 49 (1): 46–76. doi:10.1002/anie.200903708. ISSN 1433-7851.
- ↑ Stephan, D. W.; Erker, G. (2017). "Frustrated Lewis pair chemistry". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. Royal Society. 375 (2101): 20170239. Bibcode:2017RSPTA.37570239S. doi:10.1098/rsta.2017.0239. ISSN 1364-503X. PMC 5540845. PMID 28739971.
- ↑ Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. (2006). "Reversible, Metal-Free Hydrogen Activation". Science. 314 (5802): 1124–1126. Bibcode:2006Sci...314.1124W. doi:10.1126/science.1134230. ISSN 0036-8075. PMID 17110572.
- ↑ Birkmann, B.; et al. (2010). "Frustrated Lewis Pairs and Ring-Opening of THF, Dioxane, and Thioxane". Organometallics. 29: 5310–5319. doi:10.1021/om1003896.
{{cite journal}}: Explicit use of et al. in:|first1=(help)
