High School Chemistry/The Periodic Table and Electron Configurations
With what we have already discussed, you might realize that just as electron configurations can be used to explain the shape and organization of the Periodic Table, the shape and organization of the Periodic Table can, in turn, be used to predict electron configurations. In fact, if you can locate an element on the Periodic Table, you can use the element's position to figure out the energy level of the element's valence electrons. Furthermore, an element's position on the Periodic Table tells you the sublevel of the element's highest energy valence electrons. In this lesson, we'll take a close look at how the Periodic Table relates to the electron configurations.
Lesson Objectives
[edit | edit source]- Relate an element's position in the Periodic Table to the energy level of its valence electrons (excluding transition metals, lanthanides, and actinides).
- Relate an element's position in the Periodic Table to the sublevel of its highest energy valence electrons.
- Explain why there are only two elements in the first row of the Periodic Table.
Rows Across on the PT are Consistent With the Energy Level in An Atom
[edit | edit source]First, let's try to figure out what we can learn from an element's row or period in the Periodic Table.
The figure below shows how the different rows in the Periodic Table are numbered. The transition metals and the lanthanides and actinides have been omitted.

To understand what this means in terms of an element's electron configuration, let's consider the Group 1A metals. If we write the electron configuration for the Group 1A metal from each row of the Periodic Table, we have:
row 2 Li: row 3 Na: row 4 K: row 5 Rb: row 6 Cs: row 7 Fr:
Do you see any pattern? For Group 1A metals, it seems that element's row corresponds to the energy level of that element's valence electron. Lithium (Li), for instance, is found in row 2 of the Periodic Table, and its valence electron is found in the n = 2 energy level. Cesium (Cs) is found in row 6 of the Periodic Table, and its valence electron is found in the n = 6 energy level. Let's see if this same pattern holds for Group 2A metals:
row 2 Be: row 3 Mg: row 4 Ca: row 5 Sr: row 6 Ba: row 7 Ra:
For Group 2A metals, the same rule applies! Magnesium (Mg) is found in row 3 of the Periodic Table, and its valence electrons are found in the n = 3 energy level. Similarly, Radium (Ra) is found in row 7 of the Periodic Table, and its valence electrons are found in the n = 7 energy level.
So far so good – but does the same pattern apply to the Group 3A–8A elements (also known as Groups 13–18). Let's find out by writing the electron configuration for the Group 3A element in each row.
row 2 B: row 3 Al: row 4 Ga: row 5 In: row 6 Tl:
Even though the valence electrons in Group 3A elements are found in both s and p orbitals, it turns out that an element's row still corresponds to the energy level of that element's valence electrons. For example, Gallium (Ga) is found in row 4 of the Periodic Table, and its valence electrons are found in the n = 4 energy level. Likewise, Thallium (Tl) is found in row 6 of the Periodic Table, and its valence electrons are found in the n = 6 energy level.
It really does seem as if we can predict the energy level of an element's valence electrons using the row number for that element in the Periodic Table. Let's try one last example, though, just to be sure by writing the electron configuration for the Group 7A element in each row.
row 2 F: row 3 Cl: row 4 Br: row 5 I: row 6 At:
Once again, an element's row can be used to determine the energy level of that element's valence electrons. Chlorine (Cl), for instance, is found in row 3 of the Periodic Table, and its valence electrons are found in the n = 3 energy level. Similarly, Iodine (I) is found in row 5 of the Periodic Table, and its valence electrons are found in the n = 5 energy level.
You should make note of one final point when it comes to energy levels and how they relate to the Periodic Table. Our rule for determining the energy level of an element's valence electrons using the element's row in the Periodic Table works for Group 1A–8A elements. This rule doesn't apply, however, to the Group 1B–8B (also known as Groups 3–12) elements. The elements in that lower portion of the Periodic Table (the middle portion of the Periodic Table "blacked out" in the first figure) are known as transition metals. They behave differently, and you can't apply the same rules to them as far as valence electrons are concerned. The same goes for the isolated lower portion of the Periodic Table (also "blacked out" in the figure of the Periodic Table above). This block contains elements known as lanthanides and actinides. Like transition metals, lanthanides and actinides do not obey the same rules as the Group 1A–8A elements when it comes to valence electrons and valence electron energy levels.
Hydrogen and Helium Occupy the First Period
[edit | edit source]You probably noticed that, in the last section, we didn't mention the first row at all. Instead, we always started from row 2. The first row in the Periodic Table is a "special row" for several reasons. To begin with, the first row of the Periodic Table contains only two elements – hydrogen and helium. Can you figure out why there are only two elements in the first row?
According to what we learned in the last section, an element's row number corresponds to the energy level of that element's valence electrons. Therefore, the first row must contain elements with valence electrons in the n = 1 energy level. By now you should know that there is only one orbital in the n = 1 energy level. That orbital, of course, is the 1s orbital. Hydrogen has one valence electron in the 1s orbital, (its electron configuration is 1s1), and helium has two valence electrons in the 1s orbital (its electron configuration is 1s2). Since it's impossible to fit more than two electrons into the 1s orbital, atoms with a total of three or more electrons must have valence electrons in an energy level with n = 2 or greater. Clearly, then, atoms with a total of three or more electrons do not belong in the first row of the Periodic Table.
The first row of the Periodic Table is also special because its elements have special properties. Hydrogen, for example, is not a metal like the rest of the Group 1A elements. Instead, hydrogen atoms react with each other and form what's known as hydrogen gas, H2. As was mentioned in the last lesson, some scientists will put hydrogen in a category all by itself, rather than including it at the top of the 1A column. We won't do that here, but you should always keep in mind the fact that hydrogen is "different", and that you shouldn't compare the hydrogen's chemical properties with the chemical properties of the other Group 1A elements.
Helium is also a special atom. You might wonder why helium appears at the far right-hand side of the Periodic Table, rather than right next to hydrogen. Again, helium's placement in the Periodic Table reflects its special chemical properties. Earlier you learned that Group 8A elements were "inert" and that includes helium. Even though helium only has two valence electrons, while the rest of the Group 8A elements have eight valence electrons, helium is placed on the top of the 8A column since helium's chemical behavior is similar to the chemical behavior of the other noble gases because it has a completed outer energy level.
The s Sublevel Block on PT
[edit | edit source]So far we know that, with the exception of transition metals, lanthanides and actinides, we can use the row in which an element is found to determine the energy level of that element's valence electrons. Can the organization of the Periodic Table, and the placement of an element within the Periodic Table, tell us anything else about the elements electron configuration? The answer is – "yes". Remember that the highest energy valence electrons in Group 1A and Group 2A elements are always in s orbitals. In fact, the only valence electrons in Group 1A and Group 2A elements are in s orbitals! Lithium, (Li) for instance, has the electron configuration 1s22s1. Notice that lithium's single valence electron is in an s orbital. Similarly, magnesium (Mg) has the electron configuration 1s22s22p63s2. Again, notice that magnesium's two valence electrons are in an s orbital. Since all of the valence electrons in Group 1A and Group 2A elements exist in s orbitals, the first two columns of the Periodic Table (columns 1A and 2A) are known as the "s sublevel block". The s sublevel block is shown in the figure below. Notice that the s sublevel block consists of all of the metals from Li down to Fr in column 1A, and all of the metals from Be down to Ra in column 2A. Hydrogen is not included in the s sublevel block, again, because of its special properties.

The p Sublevel Block on PT
[edit | edit source]What can we say about the valence electrons in Group 3A – Group 8A elements? In particular, what can we say about the highest energy valence electrons? If you look carefully, you'll notice that for Group 3A – Group 8A (or if you prefer, Groups 13–18) elements, the highest energy valence electrons are always in p orbitals. Boron, (B) for instance, has the electron configuration 1s22s22p1. While boron has both 2s and 2p valence electrons, the 2p valence electrons are higher in energy. Similarly, Krypton (Kr) has the electron configuration 1s22s22p63s23p64s23d104p6. Again, while krypton has both 4s and 4p valence electrons, the 4p valence electrons are higher in energy. Since the highest energy valence electrons in Group 3A Group 8A elements exist in p orbitals, the final six columns of the Periodic Table (columns 3A through 8A) are known as the "p sublevel block". The p sublevel block is shown in the figure below. Additionally, as illustrated in the figure below the p sublevel block consists of all of the elements from B down to Tl in column 3A, all of the elements from C down to Pb in column 4A, all of the elements from N down to Bi in column 5A, all of the elements from O down to Po in column 6A, all of the elements from F down to At in column 7A and, finally, all of the elements from Ne down to Rn in column 8A. Helium is not included in the p sublevel block, which should make sense, since helium has no p electrons!

Just as the Periodic Table has an s sublevel block and a p sublevel block, it also has a d sublevel block and an f sublevel block. Defining valence electrons in the d and f sublevel blocks can be more difficult but, in general, most of the high energy valence electrons in the d sublevel block are in d orbitals while most of the high energy valence electrons in the f sublevel block are in f orbitals.
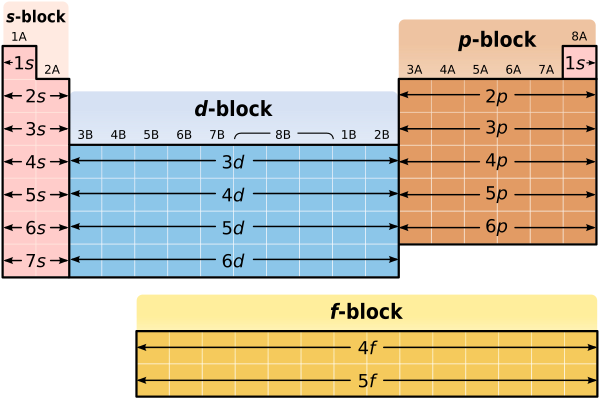
Earlier you learned that the Periodic Table was a convenient way to summarize all of the information that scientists know about the different elements found in our world. The Periodic Table probably even looked funny to you, because you had no way of understanding what its shape and organization meant. Now that we've discussed electron orbitals and electron energy levels, though, the Periodic Table shouldn't seem so strange anymore. In fact, the shape of the Periodic Table actually reflects the way in which electrons are organized in atoms of the different elements.
Lesson Summary
[edit | edit source]- For Group 1A, 2A, 3A, 4A, 5A, 6A, 7A and 8A elements, the row in which an element is found corresponds to the energy level of that element's valence electrons. For example, lithium (Li) is in row 2 and its valence electrons are in the n = 2 energy level.
- You can predict the energy level of an element's valence electrons using the element's row number in the Periodic Table.
- Elements in the first row have special properties.
- Hydrogen is not an alkali metal, and is usually found as a gas.
- Helium is a noble gas, and exhibits chemical properties similar to the other noble gases found in Group 8A.
- The Periodic Table can be divided into s, d, p, and f sub level blocks.
- For elements in the s sublevel block, all valence electrons are found in s orbitals.
- For elements in the p sub level block, the highest energy valence electrons are found in p orbitals.
Review Questions
[edit | edit source]- Use the Periodic Table to determine the energy level of the valence electrons in each of the following elements.
- (a) B
- (b) Ga
- (c) Rb
- (d) At
- (e) He
- Fill in the blanks:
- (a) B is in the __ level block of the Periodic Table
- (b) Sr is in the __ level block of the Periodic Table
- (c) Fe is in the __ level block of the Periodic Table
- (d) Cs is in the __ level block of the Periodic Table
- (e) O is in the __ level block of the Periodic Table
- Use the Periodic Table to determine the energy level and sublevel of the highest energy electrons in each of the following elements:
- (a) N
- (b) Ca
- (c) Rb
- (d) P
- (e) In
- Decide whether each of the following statements is true or false.
- (a) Li has valence electrons in the n = 1 energy level.
- (b) Si has valence electrons in the n = 3 energy level.
- (c) Ga has valence electrons in the n = 3 energy level.
- (d) Xe has valence electrons in the n = 5 energy level.
- (e) P has valence electrons in the n = 2 energy level.
- Match the element to the sublevel block it is found in:
(a) C i. s sublevel block (b) Cs ii. p sublevel block (c) Ce iii. d sublevel block (d) Cr iv. f sublevel block
- The first row of the Periodic Table has:
- (a) 1 element
- (b) 2 elements
- (c) 3 elements
- (d) 4 elements
- (e) 5 elements
- Use the Periodic Table to determine which of the following elements has the highest energy valence electrons.
- (a) Sr
- (b) As
- (c) H
- (d) At
- (e) Na
- Use the Periodic Table to determine which of the following elements has the lowest energy valence electrons.
- (a) Ga
- (b) B
- (c) Cs
- (d) Bi
- (e) Cl
- Which energy level does the first row in the d sublevel block correspond to?
Vocabulary
[edit | edit source]- d sub level block
- The elements in the Periodic Table in columns 1B through 8B (also known as transition metals).
- f sub level block
- The elements in the lanthanide and actinide rows of the Periodic Table.
- inert
- Non-reactive.
- lanthanides and actinides
- Elements in the f sub level block of the Periodic Table. The highest energy electrons in lanthanides and actinides are found in f orbitals.
- noble gases
- Group 8A elements. These are elements found in the eighth column of the Periodic Table. They are inert, which means that they are very non-reactive.
- p sublevel block
- The elements in the Periodic Table in columns 3A through 8A (excluding helium). The highest energy valence electrons for elements in the p sub level block are in p orbitals.
- s sublevel block
- The elements in the Periodic Table in columns 1A and 2A (excluding hydrogen). All valence electrons for elements in the s subblevel block are in s orbitals.
- transition metals
- Elements in the d sublevel block (columns 1B through 8B) of the Periodic Table. The highest energy electrons in transition metals are found in d orbitals.
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License






















