Chemistry 101/Elements and Atoms: The Basics of Chemistry/Purpose of The Periodic Table
History of The Periodic Table(s)[edit | edit source]
One of the original periodic tables was a creation of Dmitri Mendeleev, a Russian scientist.
After becoming a teacher, Mendeleev wrote a definitive two-volume textbook at that time: Principles of Chemistry (1868–1870). As he attempted to classify the elements according to their chemical properties, he noticed patterns that led him to postulate his Periodic Table. Mendeleev was unaware of the other work on periodic tables going on in the 1860s. He first made a small table following a particular pattern, and by adding additional elements following this pattern, developed his extended version of the periodic table, pictured here.[1]
As you can see the table consists of rows, and columns. In the case of a periodic table, the rows are referred to as periods, and the columns are referred to as groups. This table is very similar to the modern periodic table. In the modern periodic table there are seven periods, and eighteen groups. The labeling of the groups in the table can vary from country to country. You will see more on this soon.
Purpose of The Periodic Table[edit | edit source]
Divisions of The Periodic Table[edit | edit source]
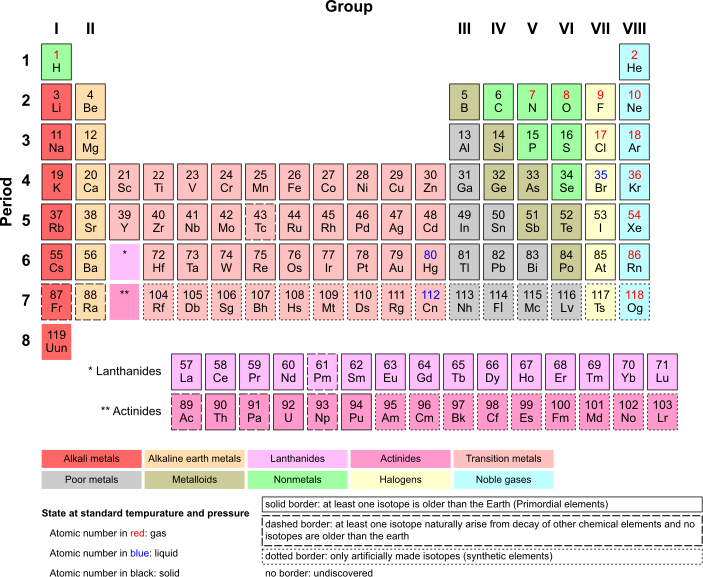 Periodic Table Roman numerals for A groups...
Periodic Table Roman numerals for A groups...
How Chemists use the Periodic Table[edit | edit source]
Atomic numbers and subatomic particles[edit | edit source]
References[edit | edit source]
- ↑ Dmitri Mendeleev(Wikipedia), Wikipedia.org

