Biomedical Engineering Theory And Practice/Bioelectric phenomena
'See also Wikipedia,Electrophysiology.
Electrophysiology is the branch of the biomedical engineering dealing with the study of electric activity in the body. Electrophysiology includes the study of the production of electrical activity and the effects of that electrical activity on the body. It involves measurements of voltage change or electrical current flow by electrodes in various systems, from single ion channel proteins to single neurons (particularly action potentials) and whole tissues like the heart.
Membrane Potential[edit | edit source]

Our Body is electrically neutral but our body cells are surrounded by a membrane made up of a lipid bilayer with proteins embedded in it. The membrane have a role as an insulator and a diffusion barrier to the movement of ions. Ion transporter/pump proteins push ions across the membrane to get concentration gradients across the membrane, and ion channels allow ions to move across the membrane down those concentration gradients. Ion pumps and ion channels are electrically same to a set of batteries and resistors in the membrane. Within membrane and outside membrane, one charge may predominates. Opposite charges try to attract and energy must be used to separate them. Conversely when opposite charges bring together, energy is liberated. Therefore separated electrical charges have potential energy.
In electrically active tissue, voltage can be measured by inserting an electrode at two point, for instance one inside and one outside the cell, and is called the potential difference or difference in electrical potential. Voltage is the ability to drive an electric current across a resistance. Flow of electrical charge from one point to another is called current. The amount of charge moving between two points depends on voltage and resistance (hindering of flow of charge). So, the simplest definition of a voltage is given by Ohm's law:
where I is the current through the conductor in units of amperes, V is the potential difference measured across the conductor in units of volts, and R is the resistance of the conductor in units of ohms. Current is proportional to voltage (the greater the voltage, the greater the current) and is inversely proportional to resistance (the greater the resistance, the less the current).
In the body, electrical currents correspond to the flow of ions across cellular membranes and any resistance is provided by the membrane themselves. Various ion channels occur in the plasma membranes and classified as being either passive or active:
- Passive (leakage) channels are always open
- Active (gated) channels that are made up of one or more proteins capable of undergoing changes to open or close
- Chemically gated channels
- Voltage-dependent channels
- Other gated channels
Ions move along chemical gradients (due to diffusion) and along electrical gradients (move toward an opposite charge); therefore ions flow along electrochemical gradients.
In non-excitable cells, and in excitable cells in their baseline states, the membrane potential has a relatively steady value, called the resting potential. The opening and closing of ion channels can cause a departure from the resting potential. This is called a depolarization if the interior voltage moves to a more positive membrane potential, or a hyperpolarization if the interior voltage becomes more negative . In excitable cells, a sufficiently large depolarization can make an action potential, in which the membrane potential changes rapidly and clearly for a short time (on the order of 1 to 100 milliseconds), often reversing its polarity.
Changes in membrane potential (specially if induced by activation of ligand-gated channels) are also defined as either:
- Excitatory: membrane depolarization
- Inhibitory: membrane hyperpolarization
Transport Membrane[edit | edit source]

Membrane transport means the movement of particles (solute) across or through a membranous barrier[1] In the cell, these membranous barriers is composed of a phospholipid bilayer. The phospholipids orient themselves automatically so that the hydrophilic (polar) heads are nearest the extracellular and intracellular mediums, and the hydrophobic (non-polar) tails align between the two hydrophilic head groups like the principle of the soap in the water.
Membrane transport depends on the permeability of the membrane, transmembrane solute concentration, and the size and charge of the solute. [1]Solute particles can pass through the membrane via three mechanisms: passive, facilitated, and active transport. Some of these transport mechanisms need the input of energy and use of a transmembrane protein, whereas other mechanisms do not accept secondary molecules.[2]
Passive transport[edit | edit source]
Passive transport rely on the concentration gradient, hydrophobicity, the size and charge of the solute. In passive transport, small uncharged solute particles diffuse across the membrane until both sides of the membrane have reached an equilibrium that is similar in concentration. [3]
Fick's first law[edit | edit source]
Fick's first law could be used to describe the passive transport of molecules across membranes.
where
- is the "diffusion flux" [(amount of substance) per unit area per unit time], for example .
- is the diffusion coefficient or diffusivity in dimensions of [length2 time−1], for example
- (for ideal mixtures) is the concentration in dimensions of [amount of substance per unit volume], for example
- is the position [length], for example
Facilitated transport[edit | edit source]
Facilitated diffusion is a kind of passive transport mediated by transport proteins imbedded within the cellular membrane.[4]
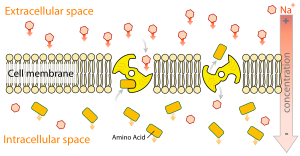
Active transport[edit | edit source]
Active transport is the movement of particles through a transport protein from low concentration to high concentration at the expense of metabolic energy.[4]
Primary active transport[edit | edit source]

Primary active transport directly uses metabolic energy to transport molecules across a membrane. Most of the enzymes that perform this type of transport are transmembrane ATPases.Based on the transport mechanism as well as genetic and structural homology, These ATP-dependent ion pumps can be classified into four types:[5]
- P-type ATPase: sodium potassium pump, calcium pump, proton pump
- F-ATPase: mitochondrial ATP synthase, chloroplast ATP synthase
- V-ATPase: vacuolar ATPase
- ABC (ATP binding cassette) transporter: MDR, CFTR, etc.
The P-, F- and V-types only transport ions, while the ABC superfamily also transports small molecules.
Secondary active transport[edit | edit source]
Secondary active transport is known as coupled transport or co-transport as energy is used to transport molecules across a membrane; however, compared to primary active transport, there is no direct coupling of ATP; instead, the electrochemical potential difference by pumping ions out of the cell is used.[6]
In August 1960, in Prague, Robert K. Crane presented for the first time his cotransport mechanism for intestinal glucose absorption.[7] Cotransporters can be divided as symporters and antiporters depending on whether the substances move in the same or opposite directions.
- symporters: Both molecules are transported into the same direction across a membrane.
- antiporters: Two species of ion or other solutes are pumped in opposite directions across a membrane.
Ion Channels[edit | edit source]

Ion channels are pore-forming membrane proteins whose functions is establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume.[8] [9] Well-known laboratory techniques for ion channels could be voltage clamp, patch clamp techniques.
Ion channels are different from other ion transporter proteins:[10]
- The rate of ion transport through the channel is very high (often 106 ions per second or above).
- Ions pass through channels down their electrochemical gradient, which is a function of ion concentration and membrane potential, "downhill", without the input of metabolic energy (e.g. Adenosine triphosphate, active transport mechanisms, co-transport mechanisms).
Ion channels are located within the plasma membrane of over 300 types of cells and many intracellular organelles. They are often described as narrow, water-filled tunnels that accept only specific type ions. This characteristic is called selective permeability. Ion channels are integral membrane proteins, formed as assemblies of several proteins. Such "multi-subunit" assemblies usually make a circular arrangement of identical or homologous proteins closely packed around a water-filled pore through the plane of the lipid bilayer membrane[11][12] .
Ion channels are synthesized and inserted into the membrane of the endoplasmic reticulum. They are glycosylated in the Golgi, and transported and inserted into target membranes by membrane fusion. They are controlled by phosphorylation, trafficking, ubiquitination, reversible interactions with other signaling proteins and second messengers, proteolytic cleavage, and other modifications. Ion channels are related to various biological processes that cause rapid changes in cells, such as muscle contraction, epithelial transport of nutrients and ions, T-cell activation and pancreatic beta-cell insulin release. Ion channels are a frequent target in order to find out the new drugs.[13][14][15]
Voltage-gated Ion Channels[edit | edit source]
Voltage-gated Ion Channels are protein channels that can be opened or closed in response to changes in the electric potential across a cell membrane. They have an important role in excitable neuronal and muscle tissues, causing a rapid and coordinated depolarization in response to triggering voltage change. They generally consist of several subunit proteins which make a pore according to their electrochemical gradients.

Examples include:
- the sodium and potassium voltage-gated channels of nerve and muscle.
- the voltage-gated calcium channels that play a role in neurotransmitter release in pre-synaptic nerve endings.
- the voltage-gated proton channels in phagocytes during the "respiratory burst."
The process in the sodium and potassium voltage-gated channels is follows:
- Depolarization of the cell interior causes the protein channel to move, inducing a conformational change such that ions may flow through the channel (the open state).
- The Na+ ions flood into the cell (along their concentration gradient).
- The delayed rectifier opens and the Na+ V-gated channel inactivates.
- K+ ions flood out of the cell (along their concentration gradient)
- The Na+/K+ pump uses ATP to restore the concentration gradients
Ligand-gated Ion Channels[edit | edit source]
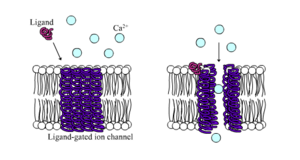
Ligand-gated ion channels are protein channels which open to accept ions such as Na+, K+, Ca2+, or Cl- to pass through the membrane in response to the binding of a chemical messenger like ligand[17],neurotransmitter and so on.[18] These proteins typically consist of at least two domains: a transmembrane domain which includes the ion pore, and an extracellular domain which includes the ligand binding location[19]. LGICs are categorized into three superfamilies: the cysteine-loop channels, ionotropic glutamate channels and the purinergic receptor channels.They are usually pentameric with each subunit including 4 transmembrane helices constituting the transmembrane domain, and a beta sheet sandwich type, extracellular, N terminal, ligand binding domain.[20] The cysteine-loop channels bind to the chemical messenger such as the chemical neurotransmitter acetylcholine (ACh), glycine (gly), gamma-aminobutyric acid (GABA), or serotonin (5-HT). The ionotropic glutamate receptors (gluR) are tetramers of homologous subunits, forming a central pore strikingly similar to the voltage-gated superfamily, but with reverse topological orientation in the membrane. The purinergic receptor channels (P2XR) is opened by extracellular nucleotides such as ATP.
G protein-gated Ion Channels[edit | edit source]
Generally, G protein-gated ion channels are specific ion channels placed in the plasma membrane of cells that are directly activated by associated proteins. G proteins also known as guanosine nucleotide-binding proteins is a heterotrimer of three subunits: α-, β-, and γ- subunits. The α-subunit (Gα) typically binds the G protein to G protein-coupled receptor. This receptor protein has a large, extracellular binding domain which will bind its respective ligands such as neurotransmitters and hormones. Once the ligand is bound to its receptor, a conformational change occurs. This conformational change in the G protein leads Gα to bind GTP. This allows to another conformational change in the G protein, causing the separation of the βγ-complex (Gβγ) from Gα.[21] At this point, both Gα and Gβγ are active and can continue the signal transduction pathway. G protein-gated ion channels are primarily found in central nervous system neurons and atrial myocytes, and affect the flow of potassium (K+), calcium (Ca2+), sodium (Na+), and chloride (Cl-) across the plasma membrane.[22]
Donnan Equilibrium[edit | edit source]

The charged particles like ions near a semi-permeable membrane sometimes fail to distribute evenly across the two sides of the membrane.[23] Usually, it happens as a different charged substance could not pass through the membrane and creates an uneven electrical charge.[24] For example, the large anionic proteins in blood plasma do not permeate to capillary walls. Because small cations are attracted, but are not bound to the proteins, small anions will cross capillary walls away from the anionic proteins more readily than small cations.
Nernst potential[edit | edit source]
The Nernst equation is used to calculate the potential of an ion of charge z across a membrane. This potential is determined using the concentration of the ion both inside and outside the cell:
For monovalent cations,z= 1:
Donnan Equilibrium & Nernst potential[edit | edit source]
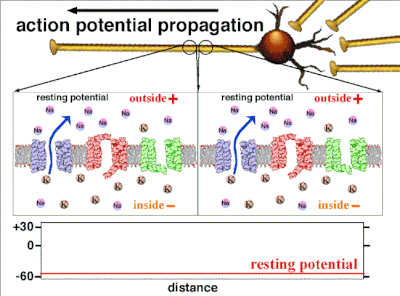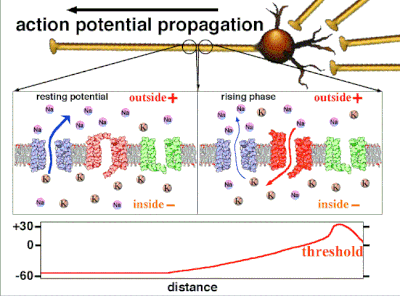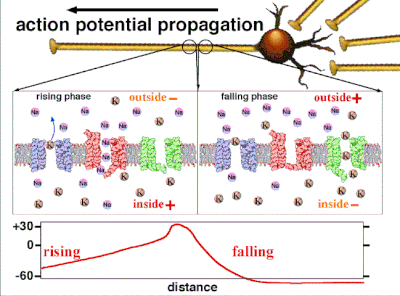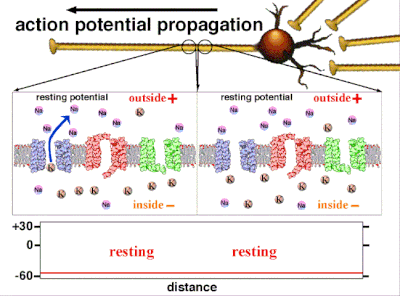
Resting Potential[edit | edit source]

The negative charge that exists intracellularly and not electrically active, is the resting potential.[25] As potassium is also the ion with the most negative equilibrium potential, usually the resting potential can be no more negative than the potassium equilibrium potential.All cells as well as neurons have a resting potential. But in neurons and in muscle fibers, the resting potential is very important, since all electrical activity will be changes from this resting potential. The resting potential can be calculated with the Goldman-Hodgkin-Katz voltage equation using the concentrations of ions as for the equilibrium potential including the relative permeabilities, or conductances, of each ionic species.
The Goldman-Hodgkin-Katz voltage equation for monovalent positive ionic species and negative:
This results in the following if we consider a membrane separating two -solutions:
- = The membrane potential (in volts, equivalent to joules per coulomb)
- = the permeability for that ion (in meters per second)
- = the extracellular concentration of that ion moles per cubic meter, to match the other SI units)
- = the intracellular concentration of that ion (in moles per cubic meter)
- = The ideal gas constant (joules per kelvin per mole)
- = The temperature in kelvins
- = Faraday's constant (coulombs per mole)
Graded potential[edit | edit source]
Graded potentials are changes in membrane potential which arise from the summation of the individual actions of ligand-gated ion channel proteins, and decrease over time and space.They do not typically involve voltage-gated sodium and potassium channels.[26]

There are 3 primary types of graded potentials:
- Receptor potentials is the transmembrane potential difference of a sensory receptor.[27] They result from transduction processes (the conversion of an energy stimulus into a electrical potential). They can be caused by the opening of either mechano- or ligand-gated channels
- Postsynaptic potentials are changes in the membrane potential of the postsynaptic terminal of a chemical synapse in neurons. Postsynaptic potentials are graded potentials, and should not be confused with action potentials although they initiate or inhibit action potentials[28]. They result from the activation of ligand-gated channels. Depolarizing are called excitatory postsynaptic potentials (EPSPs). Hyperpolarizing are called inhibitory postsynaptic potentials (IPSPs).The ionic basis of postsynaptic potentials is thought to neurotransmission.
- End plate potenials are chemically induced change in electric potential of the motor end plate,the part of the muscle-cell membrane that lies opposite the terminal of a nerve fibre at the neuromuscular junction[29]. They result from the activation of ligand-gated channels.
Action potential[edit | edit source]
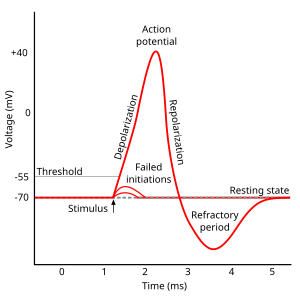
In physiology, an action potential is the brief (about one-thousandth of a second) reversal of electric polarization of the membrane of a excited cells such as nerve cell (neuron), muscle cell, endocrine cell and so on.[30]. In neurons, an action potential happens when a neuron sends information down an axon, away from the cell body. In other types of cells, they activate intracellular processes. In muscle cells, for example, an action potential produces the contraction required for all movement. In beta cells of the pancreas, they induce release of insulin.[31] Action potentials in neurons are also known as "nerve impulses" or "spikes", and the temporal sequence of action potentials generated by a neuron is called its "spike train". A neuron that emits an action potential is often said to "fire"[32].
Action potentials are generated by specific voltage-gated ion channels embedded in a cell's plasma membrane.[33] These channels are shut when the membrane potential is near the resting potential of the cell, but stimulation of the cell by neurotransmitters or by sensory receptor cells partially opens channel-shaped protein molecules in the membrane. Sodium diffuses into the cell, shifting that part of the membrane toward a less-negative polarization. If this local potential reaches a critical state called the threshold potential (measuring about −60 mV), sodium channels open completely. Sodium floods inside the cell quickly depolarizes to an action potential of about +55 mV. The declining phase of the action potential is from the closing of sodium channels and the opening of potassium channels, which let a charge about equal to the stage which the cell leaves in the form of potassium ions. Therefore, protein transport molecules pump sodium ions out of the cell and potassium ions in. This restores the original ion concentrations and the membrane is ready for a new action potential.
Biomagnetism[edit | edit source]
Biomagnetism is the phenomenon of magnetic fields created by living organisms; it is a subset of bioelectromagnetism. It examines the electric, electromagnetic, and magnetic phenomena which arise in biological tissues[34].
Maxwell Equations[edit | edit source]
Maxwell's equations describe how electric charges and electric currents create electric and magnetic fields. Therefore, when there are bioelectric fields there always are also biomagnetic fields, and vice versa (Maxwell, 1865)
| Name | Differential Equations | Integral form |
|---|---|---|
| Gauss's law for electricity | ||
| Gauss' law for magnetism (absence of magnetic monopoles): |
||
| Faraday's law of induction
(Maxwell-Faraday equation): |
||
| Ampère's law (with Maxwell's extension): |
where
- is the electric field(volt per metre).
- is the magnetic field strength(ampere per metre)
- is the electric displacement field(coulomb per square metre)
- is the magnetic flux density also called the magnetic induction(tesla, or equally, weber per square metre)
- is the freeelectric charge density. It does not count the dipole charges bound in a material.(coulomb per cubic metre)
- is the free current density.It does not count polarization or magnetization currents bound in a material.(ampere per square metre)
- is the differential vector element of surface area A, with very small magnitude and direction normal to surface S(square meters)
- is differential element of volume V enclosed by surface S(cubic meters)
- is differential vector element of path length tangential to contour C enclosing surface c(meters_
- is instantaneous velocity of the line element defined above (for moving circuits)(meters per second)
- is the divergence operator (SI unit: 1 per metre),
- is the curl operator (SI unit: 1 per metre).
Reciprocity[edit | edit source]
Table. Measurement of Fields[34]
| Bioelectricity | Bioelectromagnetism(Biomagnetism) | Biomagnetism |
|---|---|---|
| Neural cells | ||
| electroencephalography (EEG) | magnetoencephalography (MEG) | |
| electroneurography (ENG) | magnetoneurography (MNG) | |
| electroretinography (ERG) | magnetoretinography (MRG) | |
| Muscle cells | ||
| electrocardiography (ECG) | magnetocardiography (MCG) | |
| electromyography (EMG) | magnetomyography (MMG) | |
| Other tissue | ||
| electro-oculography (EOG) | magneto-oculography (MOG) | |
| electronystagmography (ENG) | magnetonystagmography (MNG) | |
| magnetopneumogram | ||
| magnetohepatogram |
Volume Conductor Theory[edit | edit source]
Volume conductor models are a basis for source analysis in Magnetocardiography (MCG), Electrocardiography (ECG),Electroencephalography (EEG) and Magnetoencephalography (MEG). Volume conduction in bioelectromagnetism, can be defined as the transmission of electric or magnetic fields from an electric primary current source through biological tissue towards measurement sensors. Volume conductor means a continuously conductive medium.The brain, meninges, skull, and scalp make a volume conductor throughout which currents created by ionic flow can be measured.

For instance,in Electrocardiogram(ECG), it is possible to place electrodes on the body surface and measure cardiac potentials as the body behaves as a conductor of the electrical currents by the heart. In the resting, polarized state, no potential difference would exist between the positive and negative electrodes(i.e., isoelectric - flat red line). Within ventricles, when the left side of the tissue becomes depolarized (representing firing of the SA node), a wave of depolarization starts to spread across the ventricles. During this time, some part temporarily remains positive on the outside (polarized) and while some is negative (depolarized); thus, there is a separation of charges which induce a potential difference between the two electrodes. As the wave of depolarization moves toward the positive electrode, conventionally, a positive voltage (upward deflection) is recorded. The voltage reaches its maximal positive value when half the part is depolarized. Once the entire ventricle mass is depolarized (all cells negative on outside), there is no longer be a potential difference and the voltage is the same to the polarized state. When repolarization occurs, starting first with the left side ( SA nodal region) then moving across the ventricle, there will once again be both positive and negative charges on the surface of the ventricle, but this time, the negative charges will be closest to the positive electrode. The wave of repolarization sweeping across the ventricle away from the negative electrode and toward the positive electrode causes, conventionally, a negative voltage (upward deflection in ventricles. It is different according to the part.) to occur. Finally, when all of the cells are repolarized, the measured voltage difference will once again be the same to the initial stage until another wave of depolarization occurs.
Volume Conductor Modeling[edit | edit source]
The Human Body as a Volume Conductor[edit | edit source]

The human body would be thought as a resistive, piecewise homogeneous and linear volume conductor. Most of the tissue is isotropic. The muscle is, however, strongly anisotropic, and the brain tissue is anisotropic,too.
Resistivity for various tissues
| Tissue | Resistivity() | Reference |
|---|---|---|
| Bladder | 4.41 | Matthew Christensen,2010[35] |
| Blood | 1.6 | Geddes and Sadler,1973[36] |
| Bone | 12400 | Matthew Christensen,2010[35] |
| Bone | 25 | Geddes and Sadler,1973[36] |
| Bone(longitudinal) | 177 | Rush and Driscoll, 1969[37] |
| Bone(circumferential) | 15 | Saha and Williams, 1992[37] |
| Brain(gray matter) | 2.2 | Rush and Driscoll, 1969[37] |
| Brain(white matter) | 6.8 | Barber and Brown, 1984[38] |
| Brain(average) | 5.8 | Barber and Brown, 1984[38] |
| Breast | 3.39 | Matthew Christensen,2010[35] |
| Fat | 21.7 | Rush, Abildskov, and McFee, 1963[39] |
| Heart | 1.75 | Matthew Christensen,2010[35] |
| Kidney | 2.11 | Matthew Christensen,2010[35] |
| Liver | 3.42 | Matthew Christensen,2010[35] |
| Lung | 1.57 | Matthew Christensen,2010[35] |
| Heart Muscle(longitudinal) | 2.5 | Rush, Abildskov, and McFee, 1963 [39] |
| Heart Muscle(transverse) | 5.6 | Rush, Abildskov, and McFee, 1963 [39] |
| Skeletal Muscle(longitudinal) | 1.9 | Epstein and Foster, 1982[40] |
| Skeletal Muscle(transverse) | 13.2 | Epstein and Foster, 1982[40] |
Electrophysiological technique[edit | edit source]
Classical electrophysiological techniques[edit | edit source]
The principal types of electrodes are:
- simple solid conductors, such as conductive discs and needles (singles or arrays, often insulated except for the tip),
- conductive tracings on printed circuit boards, also insulated except for the tip, and
- hollow tubes filled with an electrolyte, such as glass pipettes filled with potassium chloride solution or another electrolyte solution, also for extra- or intracellular recording.
The principal sample preparations include:
- living organisms,
- excised tissue (acute or cultured),
- dissociated cells from excised tissue (acute or cultured),
- artificially grown cells or tissues, or
- hybrids of the above.
Optical electrophysiological technique[edit | edit source]

Optical electrophysiological techniques were invented to overcome the limitations of classical techniques. In classical techniques, electrical activity could be observed at approximately a single point within a volume of tissue. Classical techniques need to singularize a distributed phenomenon. Interest in the spatial distribution of bioelectric activity prompted development of molecules capable of emitting light in response to their electrical or chemical environment. Examples are voltage sensitive dyes and fluorescing proteins.
With one or more such compounds administrated via perfusion, injection or gene expression, the distribution of electrical activity may be observed and recorded. According to the development of optical instrument and computer software, three dimensional molecular level experiments become possible.
Experimental Techniques[edit | edit source]
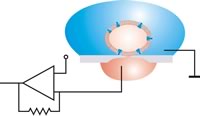
Voltage clamp technique[edit | edit source]
Through voltage clamp techniques, it became possible to understand the mechanisms involved in an action potential that is changing with voltage and time as it travels down an axon. An experimenter "clamp" the cell potential at a chosen value. In other words, it is to measure how much ionic current crosses a cell's membrane at any given voltage. It is important as a lot of the ion channels in the membrane of a neuron are voltage-gated ion channels, which open only when the membrane voltage is within a range. The data obtained from these experiments were validated by mathematical reconstruction of an action potential. [41]
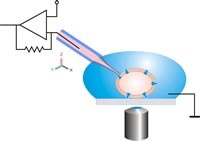
Patch clamp technique, newer voltage clamp tool since 1990[edit | edit source]


The patch clamp technique is a specialized version of the voltage clamp. The patch clamp micropipette is a micropipette with a relatively large open tip diameter(diameter of about one micron) with a polished surface rather than a sharp point. [42] [43]
This patch pipette is pressed against a cell's surface and gentle suction is applied through the microelectrode to draw a piece of the cell membrane (the 'patch') inside its tip. The suction causes the pipette to form a tight seal with the cell's membrane with an electrical resistance of several gigaohms. This configuration is the "cell-attached" mode, and it is useful for studying the activity of the ion channels in the patch of membrane.
There are multiple patch clamp configurations for various experimental observations. If more suction is applied, the small patch of membrane in the electrode tip can be displaced, leaving the electrode sealed to the rest of the cell. This "whole-cell" mode provides very stable intracellular recording. A disadvantage is that some components of the intracellular fluid could be diluted as the intracellular fluid of the cell mixes with the solution inside the recording electrode.
The "perforated patch" technique tries to minimize these problems. Instead of suction, it uses small holes on the patch with small amounts of an antifungal or antibiotic agent, such as amphothericin-B, nystatin, or gramicidin. As the antibiotic molecules diffuse into the membrane patch, they form small perforations in the membrane, allowing ions to pass through the holes freely. The problem is that it takes long to allow the antibiotic to diffuse in the cell. And the electric resistance can be higher and the patch can be rupture by the antibiotic.
This technique was developed by Erwin Neher and Bert Sakmann who received the Nobel Prize in 1991.[44]
Single electrode voltage clamp tool[edit | edit source]
Until the late 1970s the use of two microelectrodes to control the voltage in nerve and muscle cells had become a well established to measure the membrane current. But, the two-electrode voltage clamp technique faced limits in two application areas. First, as a second electrode penetrate in small cells, it caused damage. Second, in in vivo preparations the cells were commonly out of sight. Both of these problems can be resolved if the voltage clamp is implemented with a single electrode.[45]
The Single Electrode Voltage Clamp is a special purpose circuit clamping arrangement where a single microelectrode is used both to measure the membrane voltage and to pass the current necessary to control the voltage level. A fast electronic switch alternates the connection to the microelectrode between these two functions.
Planar patch clamp technique,novel automated patch clamp tool[edit | edit source]
Planar patch clamp systems is beginning to replace manual patch clamping since 1990 by research lab and companies[46]. In classical patch clamp technique, the patch pipette is moved to the cell using a micromanipulator under optical control. Relative movements between the pipette and the cell should be avoided to keep the cell-pipette connection intact. In planar patch configuration the cell is placed by suction – relative movements between cell and aperture can be excluded after sealing. An Antivibration table is not necessary. Chips are typically made from silicon, glass, PDMS, polymide. The automated patch clamp systems are usually more complex and expensive but have the advantage of parallel and hands-free operation including a large amount of data within a short time.
Computational method for experiments[edit | edit source]
Practise[edit | edit source]
Reference[edit | edit source]
- ↑ a b Reginald H. Garrett;Charles M. Grisham (2008). Biochemistry. Boston: Brooks Cole; 4th Ed. ISBN 10- 0495109355.
{{cite book}}: Check|isbn=value: length (help)CS1 maint: multiple names: authors list (link) - ↑ Stein, W. D. (1967.). The Movement of Molecules Across Cell Membranes. New York: Academic Press.
{{cite book}}: Check date values in:|year=(help) - ↑ Mathews C. K.; Van Holde, K.E; Ahern, K.G (2003). Bioquímica (3rd ed.). ISBN 84-7829-053-2.
- ↑ a b Cusslera, E.; Arisa, R.; Bhown, A. (1989). "On the limits of facilitated diffusion". Journal of Membrane Science. 2–3: 149–164.
{{cite journal}}: Text "DOI:10.1016/S0376-7388(00)85094-2" ignored (help)CS1 maint: multiple names: authors list (link) Invalid<ref>tag; name "tm4" defined multiple times with different content - ↑ Guyton, Arthur C.; Hall, John E. (2006). "Textbook of Medical Physiology", Elsevier.
- ↑ Physiologyweb,lecture note
- ↑ Crane, Robert K.; Miller, D.; Bihler, I. (1961). "The restrictions on possible mechanisms of intestinal transport of sugars". In Kleinzeller, A.; Kotyk, A. (ed.). Membrane Transport and Metabolism. Proceedings of a Symposium held in Prague, August 22–27, 1960. Prague: Czech Academy of Sciences. pp. 439–449.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ↑ Wikipedia,ion channel
- ↑ Hille, Bertil (2001) [1984]. Ion Channels of Excitable Membranes (3rd ed.). Sunderland, Mass: Sinauer Associates, Inc. p. 5. ISBN 0-87893-321-2.
- ↑ Hille, Bertil (1984). Ionic Channels of Excitable Membranes.
- ↑ Purves, (2001). "Chapter 4: Channels and Transporters". In Dale Purves, George J. Augustine, David Fitzpatrick, Lawrence. C. Katz, Anthony-Samuel LaMantia, James O. McNamara, S. Mark Williams, editors (ed.). Neuroscience (2nd ed.). Sinauer Associates Inc. ISBN 0-87893-741-2.
{{cite book}}:|editor=has generic name (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: editors list (link) - ↑ Hille B, Catterall, WA (1999). "Chapter 6: Electrical Excitability and Ion Channels". In George J Siegel, Bernard W Agranoff, R. W Albers, Stephen K Fisher and Michael D Uhler (ed.). Basic neurochemistry: molecular, cellular, and medical aspects. Philadelphia: Lippincott-Raven. ISBN 0-397-51820-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Camerino DC, Tricarico D, Desaphy JF (April 2007). "Ion channel pharmacology". Neurotherapeutics. 4 (2): 184–98. doi:10.1016/j.nurt.2007.01.013. PMID 17395128.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Verkman AS, Galietta LJ (February 2009). "Chloride channels as drug targets". Nat Rev Drug Discov. 8 (2): 153–71. doi:10.1038/nrd2780. PMC 3601949. PMID 19153558.
- ↑ Camerino DC, Desaphy JF, Tricarico D, Pierno S, Liantonio A (2008). "Therapeutic approaches to ion channel diseases". Adv. Genet. Advances in Genetics. 64: 81–145. doi:10.1016/S0065-2660(08)00804-3. ISBN 978-0-12-374621-4. PMID 19161833.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Yu FH, Catterall WA (2003). "Overview of the voltage-gated sodium channel family". Genome Biol. 4 (3): 207. doi:10.1186/gb-2003-4-3-207. PMC 153452. PMID 12620097.
- ↑ Template:DorlandsDict
- ↑ Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed. Sinauer Associates. pp. 156–7. ISBN 978-0-87893-697-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Hille B (2001) Ion Channels of Excitable Membranes, 3rd Ed. Sinauer Associates, Sunderland, Mass.
- ↑ Cascio M (2004). "Structure and function of the glycine receptor and related nicotinicoid receptors". J. Biol. Chem. 279 (19): 19383–6. doi:10.1074/jbc.R300035200. PMID 15023997.
- ↑ Stryer, Lubert; Berg, Jeremy Mark; Tymoczko, John L. (2007). Biochemistry (6th ed.). San Francisco: W.H. Freeman. ISBN 0-7167-8724-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Koyrakh L, Luján R, Colón J, Karschin C, Kurachi Y, Karschin A, Wickman K (December 2005). "Molecular and cellular diversity of neuronal G protein-gated potassium channels". J. Neurosci. 25 (49): 11468–78. doi:10.1523/JNEUROSCI.3484-05.2005. PMID 16339040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ http://www.cartage.org.lb/en/themes/Reference/dictionary/Biologie/G/13.html, retrieved 28 August 2006
- ↑ The Gibbs–Donnan Equilibrium..., D.C. Mikulecky, retrieved 28 August 2006
- ↑ An example of an electrophysiological experiment to demonstrate the importance of K+ for the resting potential. The dependence of the resting potential on the extracellular concentration of K+ is shown in Figure 2.6 of Neuroscience, 2nd edition, by Dale Purves, George J. Augustine, David Fitzpatrick, Lawrence C. Katz, Anthony-Samuel LaMantia, James O. McNamara, S. Mark Williams. Sunderland (MA): Sinauer Associates, Inc.; 2001.
- ↑ Hille 2001, pp. 169–200. "Chapter 6. Ligand-gated channels of fast chemical synapses."
- ↑ Hille, Bertil (2001). "Chapter 8. Sensory transduction and excitable cells.". Ion Channels of Excitable Membranes (3rd ed.). Sunderland, Massachusetts: Sinauer. pp. 237–268. ISBN 0-87893-321-2.
{{cite book}}: Invalid|ref=harv(help) - ↑ Wikipedia,postsynaptic potentials
- ↑ Britanica,end-plate potential (EPP)
- ↑ Britanica, action potential
- ↑ MacDonald PE, Rorsman P (February 2006). "Oscillations, intercellular coupling, and insulin secretion in pancreatic beta cells". PLoS Biol. 4 (2): e49. doi:10.1371/journal.pbio.0040049. PMC 1363709. PMID 16464129.
- ↑ Wikipedia,Action Potential
- ↑ Barnett MW, Larkman PM (June 2007). "The action potential". Pract Neurol. 7 (3): 192–7. PMID 17515599.
- ↑ a b Bioelectromagnetism - Principles and Applications of Bioelectric and Biomagnetic Fields, Oxford University Press, New York, 1995.
- ↑ a b c d e f g Matthew Christensen,WO2010126827 A2, Nov 4, 2010
- ↑ a b Geddes LA, Baker LE (1967): The specific resistance of biological material - A compendium of data for the biomedical engineering and physiologist. Med. Biol. Eng. 5: 271-93
- ↑ a b c Rush S, Driscoll DA (1969): EEG-electrode sensitivity - An application of reciprocity. IEEE Trans. Biomed. Eng. BME-16:(1) 15-22. Invalid
<ref>tag; name "Rush" defined multiple times with different content - ↑ a b Barber DC, Brown BH (1984): Applied potential tomography. J. Phys. E.: Sci. Instrum. 17: 723-33.
- ↑ a b c Rush S, Abildskov JA, McFee R (1963): Resistivity of body tissues at low frequencies. Circulation 22:(1) 40-50.
- ↑ a b Epstein BR, Foster KR (1983): Anisotropy as a dielectric property of skeletal muscle. Med. & Biol. Eng. & Comput. 21:(1) 51-5.
- ↑ Cole K.S. (1955) Ions, potentials and the nerve impulse. In Shedlovsky, T (ed.) Electrochemistry in biology ad medicine. New York, Wiley. p 121-140
- ↑ Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. (1981). "Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches". Pflügers Archiv European Journal of Physiology. 391 (2): 85–100. doi:10.1007/BF00656997. PMID 6270629.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Tanzi S, Østergaard PF, Matteucci M, Christiansen TL, Cech J, Marie R, Taboryski RJ (2012). "Fabrication of combined-scale nano- and microfluidic polymer systems using a multilevel dry etching, electroplating and molding process". Journal of Micromechanics and Microengineering. 22 (11): 115008. Bibcode:2012JMiMi..22k5008T. doi:10.1088/0960-1317/22/11/115008.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Nobel prize Medicine 1991
- ↑ The Axon Guide for Electrophysiology & Biophysics Laboratory Techniques(1993)
- ↑ http://www.nanion.de/pdf/PlanarPatchClamping.pdf











![{\displaystyle E={\frac {RT}{zF}}\ln {\frac {[{\text{ion outside cell}}]}{[{\text{ion inside cell}}]}}=2.303{\frac {RT}{zF}}\log _{10}{\frac {[{\text{ion outside cell}}]}{[{\text{ion inside cell}}]}}.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6d6f087a94da0f33a7735fa18ca8408bbca442cf)
![{\displaystyle E=-60mV\ln {\frac {[{\text{ion outside cell}}]}{[{\text{ion inside cell}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bac658923af68e11720fa4ba0e6edafc93898bcb)


![{\displaystyle E_{m}={\frac {RT}{F}}\ln {\left({\frac {\sum _{i}^{N}P_{M_{i}^{+}}[M_{i}^{+}]_{\mathrm {out} }+\sum _{j}^{M}P_{A_{j}^{-}}[A_{j}^{-}]_{\mathrm {in} }}{\sum _{i}^{N}P_{M_{i}^{+}}[M_{i}^{+}]_{\mathrm {in} }+\sum _{j}^{M}P_{A_{j}^{-}}[A_{j}^{-}]_{\mathrm {out} }}}\right)}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4168067dcf31b41af9beddcc7e9a3beba72aab8c)

![{\displaystyle E_{m,\mathrm {K} _{x}\mathrm {Na} _{1-x}\mathrm {Cl} }={\frac {RT}{F}}\ln {\left({\frac {P_{Na^{+}}[Na^{+}]_{\mathrm {out} }+P_{K^{+}}[K^{+}]_{\mathrm {out} }+P_{Cl^{-}}[Cl^{-}]_{\mathrm {in} }}{P_{Na^{+}}[Na^{+}]_{\mathrm {in} }+P_{K^{+}}[K^{+}]_{\mathrm {in} }+P_{Cl^{-}}[Cl^{-}]_{\mathrm {out} }}}\right)}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2504511122b4215bf6fa6aef42441b3da96cec09)


![{\displaystyle [ion]_{\mathrm {out} }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c5d2e7fe4863a9c797f60607f91baeab4391607b)
![{\displaystyle [ion]_{\mathrm {in} }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/23ebffa4ae9cdb7523b8a4dd8e1752a6380b22e3)