Science: An Elementary Teacher’s Guide/The Periodic Table
The Periodic Table of Elements
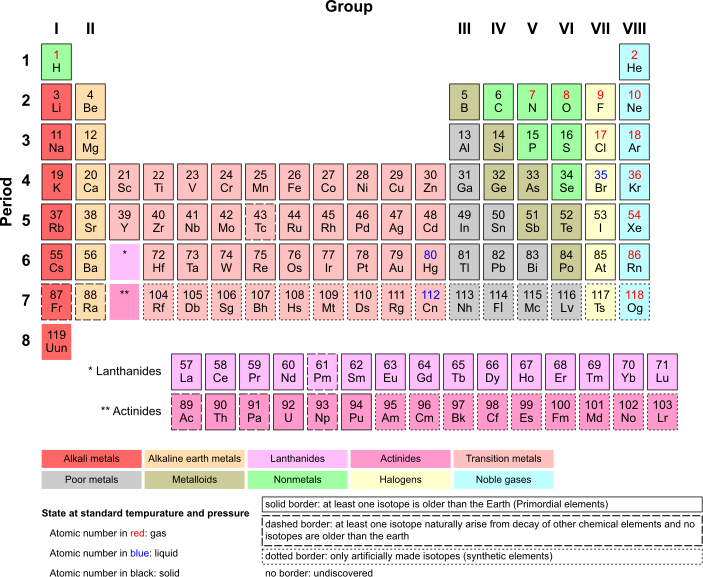
[edit | edit source]The periodic table is a chart made up of 118 elements.
The layout of the table has been exchanging and extended over time, as new elements have been discovered and new theoretical models have been developed to explain chemical behavior making the periodic table new and interesting. In 1869 Dmitri Mendeleev, published the table of elements in which the modern periodic table is based. There are 18 groups in the periodic table. Each element is in a specific location based on its atomic structure.
The atomic number of an elements relies on how many protons are found in the nucleus of an atom.
The periodic table arranges elements in periods and groups.
The periods are arranged in horizontal rows. The elements on the periodic table are arranged so that their atomic numbers increase in order from left to right.
The groups are arranged in vertical columns. Elements in the same group have similar chemical properties. Meaning they have the same amount of electrons in their outer shells.
On the far left, we have the alkali metals which are very reactive. These elements much be extracted fro compounds that contain them. Next, we have the alkaline earth metals which are reactive, but not as reactive as the alkali metals. In the middle, we have the transition metals which are nonreactive. Metals included here are iron, nickel, gold, and platinum. These metals are great conductors of heat and electricity. Halogens are located on the far right and they are extremely reactive gases. They are highly reactive with alkali metals and alkali earth metals. The metals, metalloids, gases, and nonmetals are located to the left of the halogens. The metals are solid at room temperature(except for Mercury), metalloids possess characteristics of metals and nonmetals, nonmetals are brittle solids, and nonmetals gain electrons easily. The lanthanides and actinides are located on the lower part of the Periodic Table. They are so similar it is difficult to separate them from each other. Lanthanides have high melting and boiling points and are very reactive. The actinides are all radioactive and very dense metals. Finally, to the far right we have the noble gases and they are nonreactive.