Science: An Elementary Teacher’s Guide/The Building Blocks of Matter
The Nature of Matter
[edit | edit source]Matter is the raw material of the universe. Stars, planets, mountains, oceans, and atmospheres are all made of matter. So are plants and animals—including humans and every material thing we have ever produced. Amazingly, this immense variety is generated by a limited number of chemical elements that combine in simple, well-defined ways.

Matter can be in any of the following three states: solid, liquid or gas.
- Solids can be hard or soft, but they have a definite size and shape, some examples are a rock, cotton or wood.
- Liquid, such as water, has a definite size but it has no definite shape and therefore takes the shape of its container.
- Gas, has neither a definite size nor a definite shape. It assumes both the size and shape of its container.
Most substances can change from one state to another, given the necessary temperature, but water is the only one common substance that exists in nature in all three states.
Properties of Matter
[edit | edit source]The physical properties of matter are texture, appearance, odor, color, boiling point, melting point, solubility, density, polarity and others. This physical properties describe the observations of the three states of matter mentioned before.
Cohesion and Adhesion
[edit | edit source]Cohesion refers to the attraction of molecules for other molecules of the same kind, water molecules have strong cohesive forces because of their ability to form hydrogen bonds with one another. The molecules of a solid have a much stronger cohesive attraction than those of a liquid.
Adhesion attributes to the molecules of most substances that also have an attraction for molecules of certain other substances. The water molecules have an adhesive attraction with many other materials, some examples are wood and glass, leaving such surfaces wet if water comes in contact with them.
The Building Blocks of Matter
[edit | edit source]
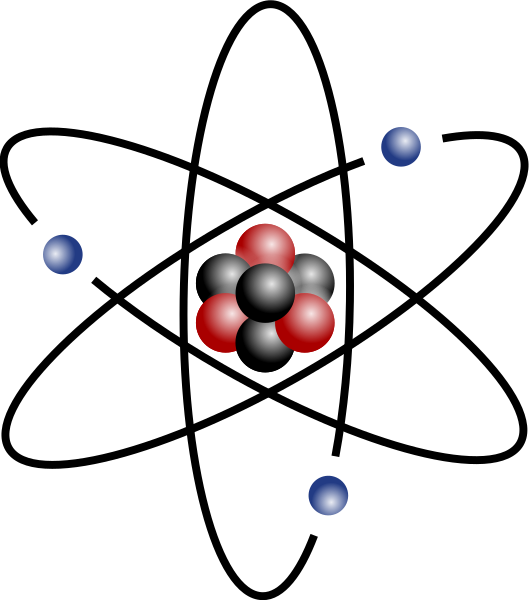
The basic facts to know about the atom are that it is made up of three basic subatomic particles: 1) electrons (negative charge) that spin in shells around a nucleus that consists of 2) protons (positive charge) and 3) neutrons (neutral charge). Generally, the number of protons and electrons balance out to make the atom have an electrically neutral charge. Electrons that are farthest away from the nucleus of an atom (valence electrons) are the ones that are most easily shared with or transferred to other atoms. The atoms that are missing an electron or share an additional electron are called ions and combine easily with other ions to make molecules.
The number of protons in an atom is called the atomic number. This number determines the element of the atom. Within an element, the number of neutrons may vary, creating the different isotopes or nuclides. For the most part, this does not affect the electrical and chemical behavior of the atom. (There is some exception with the mass of the isotope, as heavier isotopes tend to react more slowly than lighter ones.) There are some things that affect the number of protons and neutrons in the nucleus of an atom, including nuclear fission, nuclear fusion and radioactive decay. Normally, though, the number of electrons is the particle that is most easily changed, because of its lower bonding energy.
Traditionally, the atom was represented as a kind of miniature solar system. Now, scientists understand that if we could see an atom, it would look more like a fuzzy little cloud. In fact, scientists can only predict where an electron might be in its shell using the probability theory: the exact position and momentum of an electron cannot be determined simultaneously.
A colorful drawing shows the relative size of atoms, neutrons, protons and electrons. Figure 1. Atomic scale. copyright Protons and neutrons are about the same mass; however, electrons are over 1000 times lighter. How small are we talking? Well, as shown in Figure 1, we're talking very, very tiny.
atom = 1 x 10-10 meters nucleus = 1 x 10-15 to 1 x 10-14meters neutron or proton = 1 x 10-15 meters electron - not known exactly, but thought to be on the order of 1 x 10-18 meters The atom can be broken down into several, smaller subatomic particles. The three main ones are protons and neutrons, which are found in the nucleus or core of the atom, and electrons, which exist outside of the nucleus. Physicists have recently divided atoms into even smaller subatomic particles such as fermions (quarks, leptons, neutrinos, electrons) and bosons (gluons, photons, gravitrons). It is difficult (if not impossible) to determine the physical properties of something based on the number or quarks and leptons it contains. The things we see in our world (water, wood, metal, skin, teeth) are better understood and organized by using the number of protons, neutrons and electrons their atoms (and molecules) contain
Atoms
[edit | edit source]The basic building blocks that make up matter are called atoms. Sometimes two or more atoms bond together and create a molecule. A molecule is a tiny part of the substance that still has all its properties of that substance. Atoms are small but the pack a clash when their energy is released. Atoms consist of three particles: protons, neutrons, and electrons. The protons and neutrons are formed in the nucleus at the center of the atom. Protons contain a positive charge and neutrons contain a neutral charge. Electrons contain a negative charge, and they can be found in the shells around the outside of the nucleus. Mostly all atoms have the same number of electrons as protons, and that makes the atom neutral. Negative charges attract with positive charges. A positive charge will repel with another positive charge, as well a negative charge will repel with another negative charge (opposites attract).
https://www.youtube.com/watch?v=G3ImfVYSxoc
Nucleus The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The nucleus is also surrounded by an electron cloud. The diameter of a nucleus is between 1.6 fm to about 1.5 fm.
Protons are spin-½ fermions and are composed of three valence quarks,[6] making them baryons (a sub-type of hadrons). The two up quarks and one down quark of a proton are held together by the strong force, mediated by gluons.[7]:21–22A modern perspective has a proton composed of the valence quarks (up, up, down), the gluons, and transitory pairs of sea quarks. Protons have an approximately exponentially decaying positive charge distribution with a mean square radius of about 0.8 fm.[8]
Protons and neutrons are both nucleons, which may be bound together by the nuclear force to form atomic nuclei. The nucleus of the most common isotope of the hydrogen atom (with the chemical symbol "H") is a lone proton. The nuclei of the heavy hydrogen isotopes deuterium and tritium contain one proton bound to one and two neutrons, respectively. All other types of atomic nuclei are composed of two or more protons and various numbers of neutrons.
The neutron is a subatomic particle, symbol n or n0 , with no net electric charge and a mass slightly larger than that of a proton. Protons and neutrons, each with mass approximately one atomic mass unit, constitute the nucleus of an atom, and they are collectively referred to as nucleons.[5] Their properties and interactions are described by nuclear physics.
The nucleus consists of Z protons, where Z is called the atomic number, and N neutrons, where N is the neutron number. The atomic number defines the chemical properties of the atom, and the neutron number determines the isotope or nuclide.[6] The terms isotope and nuclide are often used synonymously, but they are chemical and nuclear concepts, respectively. The atomic mass number, symbol A, equals Z+N. For example, carbon has atomic number 6, and its abundant carbon-12 isotope has 6 neutrons, whereas its rare carbon-13 isotope has 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine. Other elements occur with many stable isotopes, such as tin with ten stable isotopes. Even though it is not a chemical element, the neutron is included in the table of nuclides.[7]
Within the nucleus, protons and neutrons are bound together through the nuclear force, and neutrons are required for the stability of nuclei. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered in 1932,[8] neutrons were used to induce many different types of nuclear transmutations. With the discovery of nuclear fission in 1938,[9] it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, etc., in a cascade known as a nuclear chain reaction.[6] These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Free neutrons, or individual neutrons free of the nucleus, are effectively a form of ionizing radiation, and as such, are a biological hazard, depending upon dose.[6] A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.[10] Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments.
The electron is a subatomic particle, symbol e− or β− , with a negative elementary electric charge.[8] Electrons belong to the first generation of the lepton particle family,[9] and are generally thought to be elementary particles because they have no known components or substructure.[1] The electron has a mass that is approximately 1/1836 that of the proton.[10] Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. As it is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[9] Like all matter, electrons have properties of both particles and waves: they can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer De Broglie wavelength for a given energy.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions.[11] Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer it will generate a magnetic field. Electromagnetic fields produced from other sources (not those self-produced) will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.[12] In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms.[3] Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897.[5][13][14] Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles can be totally annihilated, producing gamma ray photons.