Radioactive Waste Management/Radiation Interaction Fundamentals
There are four fundamental particles that you need to know to have a better understanding of radioactive waste. The four particles are
- alpha
- beta
- gamma
- neutron
In addition, there are a two different properties of all radiation that need to be defined half life and radioactivity.
Alpha particles (named after and denoted by the first letter in the Greek alphabet, α consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is produced in the process of alpha decay. The alpha particle can be written as , or (as it is possible that the ion gains electrons from the environment. Also, electrons are not important in nuclear chemistry). Not all helium nuclei are always considered by all authors as alpha particles. As with beta particles and gamma rays, the name used for the particle carries some mild connotations about its production process and energy.
Alpha particles, like helium nuclei, have a net spin of zero, and (due to the mechanism of their production in nuclear decay) classically a total energy of about 5 MeV. They are a highly ionizing form of particle radiation, and (when resulting from radioactive alpha decay) have low penetration depth. They are able to be stopped by a few centimeters of air, or by the skin. However, as noted, the helium nuclei which form 10-12% of cosmic rays are usually of much higher energy than those produced by radioactive decay, and are thus capable of being highly penetrating, able to traverse the human body and also many meters of dense solid shielding, depending on their energy.
The alpha decay process
[edit | edit source]Sources
[edit | edit source]When an atom emits an alpha particle, the atom's mass number decreases by four due to the loss of the four nucleons in the alpha particle. The atomic number of the atom goes down by exactly two, as a result of the loss of two protons – the atom becomes a new element. Examples of this are when uranium becomes thorium, or radium becomes radon gas due to alpha decay.
Alpha particles are commonly emitted by all of the larger radioactive nuclei such as uranium, thorium, actinium, and radium, as well as the transuranic elements. Unlike other types of decay, alpha decay as a process must have a minimum-size atomic nucleus which can support it. The smallest nuclei which have to date been found to be capable of alpha emission are the lightest nuclides of tellurium (element 52), with mass numbers between 106 and 110. The process of emitting an alpha sometimes leaves the nucleus in an excited state, with the emission of a gamma ray removing the excess energy.
In contrast to beta decay, the fundamental interactions responsible for alpha decay are a balance between the electromagnetic force and nuclear force. Alpha decay results from the Coulomb repulsion between the alpha particle and the rest of the nucleus, which both have a positive electric charge, but which is kept in check by the nuclear force.
Energy and absorption
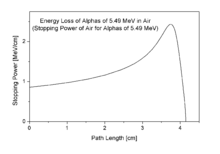
[edit | edit source]The energy of the alpha emitted is mildly dependent on the half-life for the emission process, with many orders of magnitude differences in half-life being associated with energy changes of less than 50% (see alpha decay). The energy of alpha particles emitted varies, with higher energy alpha particles being emitted from larger nuclei, but most alpha particles have energies of between 3 and 7 MeV (mega-electron-volts), corresponding to extremely long to extremely short half-lives of alpha-emitting nuclides, respectively.
This energy is a substantial amount of energy for a single particle, but their high mass means alpha particles have a lower speed (with a typical kinetic energy of 5 MeV, the speed is 15,000 km/s which is 5% of the speed of light) than any other common type of radiation (β particles, neutrons, etc.). γ rays, being an electromagnetic radiation, move at the speed of light. Because of their charge and large mass, alpha particles are easily absorbed by materials, and they can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 micrometres, equivalent to a few cells deep).
Biological effects
[edit | edit source]Because of the short range of absorption, alphas are not generally dangerous to life unless the source is ingested or inhaled, in which case, they become extremely dangerous. Because of this high mass and strong absorption, if alpha emitting radionuclides do enter the body (if radioactive material has been inhaled, ingested or injected, as with the use of Thorotrast for high-quality x-ray images prior to the 1950s), alpha radiation is the most destructive form of ionizing radiation. It is the most strongly ionizing, and with large enough doses can cause any or all of the symptoms of radiation poisoning. It is estimated that chromosome damage from alpha particles is about 100 times greater than that caused by an equivalent amount of other radiation. The powerful alpha emitter polonium-210 (a milligram of 210Po emits as many alpha particles per second as 4.215 grams of 226Ra) is suspected of playing a role in lung cancer and bladder cancer related to tobacco smoking
Not only do alphas themselves cause damage, but approximately equal ionization is caused by the recoiling nucleus after alpha emission, and this energy may in turn be especially damaging to genetic material, since the positive cations of many soluble transuranic elements which emit alphas, are chemically attracted to the net negative charge of DNA, causing the recoiling atomic nucleus to be in close proximation to the DNA.
History of discovery and use
[edit | edit source]
In the years 1899 and 1900 physicists Ernest Rutherford and Paul Villard separated radiation into three types: alpha, beta, and gamma, based on penetration of objects and ability to cause ionization. Alpha rays were defined by Rutherford by their lowest penetration of ordinary objects.

Rutherford's work also included measurements of the ratio of an alpha particle's mass to its charge, allowing him to hypothesize that alpha particles were helium nuclei (deuterium nuclei, which have the same mass to charge ratio, were not then known).[1] In 1907, Ernest Rutherford and Thomas Royds finally proved that alpha particles were indeed helium nuclei. To do this they allowed alpha particles to penetrate a very thin glass wall of an evacuated tube, thus capturing a large number of the hypothesized helium nuclei inside the tube. They then caused an electric spark inside the tube, which provided a shower of electrons which were taken up by the nuclei to form neutral atoms of a gas. Subsequent study of the spectra of the resulting gas showed that alpha particles were indeed the hypothesize helium nuclei.
Because alpha particles occur naturally, but can have energy high enough to participate in a nuclear reaction, study of them led to much early knowledge of nuclear physics. Rutherford used alpha particles emitted by radium bromide to infer that J. J. Thomson's Plum pudding model of the atom was fundamentally flawed. In Geiger-Marsden experiment Rutherford's gold foil experiment conducted by his students Hans Geiger and Ernest Marsden, a narrow beam of alpha particles was established, passing through very thin (a few hundred atoms thick) gold foil. The alpha particles were detected by a zinc sulfide screen, which emits a flash of light upon an alpha particle collision. Rutherford hypothesized that, assuming the plum pudding model of the atom was correct, the positively charged alpha particles would be only slightly deflected, if at all, by the dispersed positive charge predicted.
It was found that some of the alpha particles were deflected at much larger angles than expected (at a suggestion by Rutherford to check it) and some even bounced almost directly back. Although most of the alpha particles went straight through as expected, Rutherford commented that the few particles that were deflected was akin to shooting a fifteen inch shell at tissue paper only to have it bounce off, again assuming the "plum pudding" theory was correct. It was determined that the atom's positive charge was concentrated in a small area in its center, making the positive charge dense enough to deflect any positively charged alpha particles that came close to what was later termed the nucleus.
Note: Prior to this discovery, it was not known that alpha particles were themselves atomic nuclei, nor was the existence of protons or neutrons known. After this discovery J.J. Thompson's "plum pudding" model was abandoned, and Rutherford's experiment led to the Bohr model (named for Niels Bohr) and later the modern wave-mechanical model of the atom.
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle (an electron or a positron) is emitted. In the case of electron emission, it is referred to as beta minus (e−), while in the case of a positron emission as beta plus (e+). The emitted beta particles have a continuous kinetic energy spectrum ranging from 0 to the maximal available energy (Q), which depends on the parent and daughter nuclear states that participate in the decay. A typical Q is around 1 MeV, but it can range from a few keV to a few tens of MeV. Like the equivalence of energy of the rest mass of electron is 511 keV, the most energetic beta particles are ultrarelativistic, with speeds very close to the speed of light.
At the fundamental level (as depicted in the Feynman diagram below), this is due to the conversion of a down quark to an up quark by emission of a W- boson; the W- boson subsequently decays into an electron and an electron antineutrino.
So, unlike decay cannot occur in isolation, because it requires energy, the mass of the neutron being greater than the mass of the proton. decay can only happen inside nuclei when the value of the binding energy of the mother nucleus is less than that of the daughter nucleus. The difference between these energies goes into the reaction of converting a proton into a neutron, a positron and a neutrino and into the kinetic energy of these particles.
Electron capture (K-capture)
[edit | edit source]In all the cases where decay is allowed energetically (and the proton is a part of a nucleus with electron shells), it is accompanied by the electron capture process, when an atomic electron is captured by a nucleus with the emission of a neutrino:
energy + p + e- → n + ve
But if the energy difference between initial and final states is less than 2mec2, then decay is not energetically possible, and electron capture is the sole decay mode.
This decay is also called K-capture, because the 'inner most' electron of an atom belongs to the K-shell of the electronic configuration of the atom and this has the highest probability to interact with the nucleus.
Nuclear transmutation
[edit | edit source]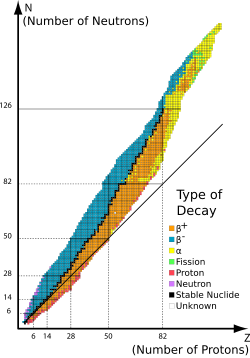
If the proton and neutron are part of an atomic nucleus, these decay processes transmute one chemical element into another. For example:
Beta decay does not change the number of nucleon s, A, in the nucleus but changes only its electric charge, Z. Thus, the set of all nuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. Among them, several nuclides (at least one) are beta stable, because they present local minima of the mass excess: if such a nucleus has (A, Z) numbers, the neighbour nuclei (A, Z−1) and (A, Z+1) have higher mass excess and can beta decay into (A, Z), but not vice versa. For all odd mass numbers A the global minimum is also the unique local minimum. For even A, there are up to three different beta-stable isobars experimentally known; for example, 96Zr, 96Mo, and 96Ru are all beta-stable, though the first one can undergo a very rare double beta decay (see below). There are about 355 known beta-decay stable nuclides total.
A beta-stable nucleus may undergo other kinds of radioactive decay (alpha decay, for example). In nature, most isotopes are beta stable, but a few exceptions exist with half-lives so long that they have not had enough time to decay since the moment of their nucleosynthesis. One example is 40K, which undergoes all three types of beta decay (and electron capture) with a half life of 1.277×109 years.
History
[edit | edit source]Historically, the study of beta decay provided the first physical evidence of the neutrino. In 1911 Lise Meitner and Otto Hahn performed an experiment that showed that the energies of electrons emitted by beta decay had a continuous rather than discrete spectrum. This was in apparent contradiction to the law of conservation of energy, as it appeared that energy was lost in the beta decay process. A second problem was that the spin of the Nitrogen-14 atom was 1, in contradiction to the Rutherford prediction of ½.
In 1920-1927, Charles Drummond Ellis (along with James Chadwick and colleagues) established clearly that the beta decay spectrum is really continuous, ending all controversies.
In a famous letter written in 1930 Wolfgang Pauli suggested that in addition to electrons and protons atoms also contained an extremely light neutral particle which he called the neutron. He suggested that this "neutron" was also emitted during beta decay and had simply not yet been observed. In 1931 Enrico Fermi renamed Pauli's "neutron" to neutrino, and in 1934 Fermi published a very successful model of beta decay in which neutrinos were produced.

Gamma radiation, also known as gamma rays (γ), is electromagnetic radiation of high frequency (very short wavelength). They are produced by sub-atomic particle interactions such as electron-positron annihilation, neutral pion decay, radioactive decay, fusion, fission or inverse Compton scattering in astrophysical processes. Gamma rays typically have frequencies above 1019 Hz, and therefore have energies above 100 keV and wavelength less than 10 picometers, often smaller than an atom. Gamma rays from radioactive decay commonly have energies of a few hundred keV, and are almost always less than 10 MeV in energy.
Because they are a form of ionizing radiation, gamma rays can cause serious damage when absorbed by living tissue, and are therefore a health hazard.
Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium Alpha and beta "rays" had already been separated and named by the work of Ernest Rutherford in 1899, and in 1903 Rutherford named Villard's distinct new radiation "gamma rays."
In the past, the distinction between X-rays and gamma rays was based on energy (or equivalently frequency or wavelength), with gamma rays being considered a higher-energy version of X-rays. However, modern high-energy (megavoltage) X-rays produced by linear accelerators ("linacs") for megavoltage treatment, in cancer radiotherapy usually have higher energy than gamma rays produced by radioactive gamma decay. Conversely, one of the most common gamma-ray emitting isotopes used in diagnostic nuclear medicine, technetium-99m, produces gamma radiation of about the same energy (140 KeV) as produced by a diagnostic X-ray machine, and significantly lower energy than therapeutic photons from linacs. Because of this broad overlap in energy ranges, the two types of electromagnetic radiation are now usually defined by their origin: X-rays are emitted by electrons (either in orbitals outside of the nucleus, or while being accelerated to produce Bremsstrahlung-type radiation), while gamma rays are emitted by the nucleus or from other particle decays or annihilation events. There is no lower limit to the energy of photons produced by nuclear reactions, and thus ultraviolet and even lower energy photons produced by these processes would also be defined as "gamma rays".[2]
In certain fields such as astronomy, gamma rays and X-rays are still sometimes defined by energy, or used interchangeably, since the processes which produce them may be uncertain.

These are produced by cosmic ray bombardment of its surface. The Sun, which has no similar surface of high atomic number to act as target for cosmic rays, cannot be seen at all at these energies, which are too high to emerge from primary nuclear reactions, such as solar nuclear fusion.[2]
Units of measure and exposure
[edit | edit source]The measure of gamma rays' ionizing ability is called the exposure:
- The coulomb per kilogram (C/kg) is the SI unit of ionizing radiation exposure, and is the amount of radiation required to create 1 coulomb of charge of each polarity in 1 kilogram of matter.
- The röntgen (R) is an obsolete traditional unit of exposure, which represented the amount of radiation required to create 1 esu of charge of each polarity in 1 cubic centimeter of dry air. 1 röntgen = 2.58×10-4 C/kg
However, the effect of gamma and other ionizing radiation on living tissue is more closely related to the amount of energy deposited rather than the charge. This is called the absorbed dose:
- The gray (Gy), which has units of (J/kg), is the SI unit of absorbed dose, and is the amount of radiation required to deposit 1 joule of energy in 1 kilogram of any kind of matter.
- The rad is the (obsolete) corresponding traditional unit, equal to 0.01 J deposited per kg. 100 rad = 1 Gy.
The equivalent dose is the measure of the biological effect of radiation on human tissue. For gamma rays it is equal to the absorbed dose.
- The sievert (Sv) is the SI unit of equivalent dose, which for gamma rays is numerically equal to the gray (Gy).
- The rem is the traditional unit of equivalent dose. For gamma rays it is equal to the rad or 0.01 J of energy deposited per kg. 1 Sv = 100 rem.
Properties
[edit | edit source]Shielding
[edit | edit source]Shielding from gamma rays requires large amounts of mass. They are better absorbed by materials with high atomic numbers and high density, although neither effect is important compared to the total mass per area in the path of the gamma ray. For this reason, a lead shield is only modestly better (20-30%) as a gamma shield than an equal mass of another shielding material such as aluminium, concrete, or soil; the lead's major advantage is its density.
The higher the energy of the gamma rays, the thicker the shielding required. Materials for shielding gamma rays are typically measured by the thickness required to reduce the intensity of the gamma rays by one half (the half value layer or HVL). For example, gamma rays that, require 1 cm (0.4″) of lead to reduce their intensity by 50%, will also have their intensity reduced in half by 4.1 cm of granite rock, 6 cm (2½″) of concrete, or 9 cm (3½″) of packed soil. However, the mass of this much concrete or soil is only 20–30% larger than that of this amount of lead. Depleted uranium is used for shielding in portable gamma ray sources, but again the savings in weight over lead is modest, and the main effect is to reduce shielding bulk.
Matter interaction
[edit | edit source]
The total absorption coefficient of aluminium (atomic number 13) for gamma rays, plotted versus gamma energy, and the contributions by the three effects. Over most of the energy region shown, the Compton effect dominates.

The total absorption coefficient of lead (atomic number 82) for gamma rays, plotted versus gamma energy, and the contributions by the three effects. Here, the photoelectric effect dominates at low energy. Above 5 MeV, pair production starts to dominate
When a gamma ray passes through matter, the probability for absorption in a thin layer is proportional to the thickness of that layer. This leads to an exponential decrease of intensity with thickness. The exponential absorption holds only for a narrow beam of gamma rays. If a wide beam of gamma rays passes through a thick slab of concrete the scattering from the sides reduces the absorption.
Here µ = ns is the absorption coefficient, measured in cm−1 and d the thickness of material in cm.
In passing through matter, gamma radiation ionizes via three main processes: the photoelectric effect, Compton scattering, and pair production.
- Photoelectric effect: This describes the case in which a gamma photon interacts with and transfers its energy to an atomic electron, ejecting that electron from the atom. The kinetic energy of the resulting photoelectron is equal to the energy of the incident gamma photon minus the binding energy of the electron. The photoelectric effect is the dominant energy transfer mechanism for x-ray and gamma ray photons with energies below 50 keV (thousand electron volts), but it is much less important at higher energies.
- Compton scattering: This is an interaction in which an incident gamma photon loses enough energy to an atomic electron to cause its ejection, with the remainder of the original photon's energy being emitted as a new, lower energy gamma photon with an emission direction different from that of the incident gamma photon. The probability of Compton scatter decreases with increasing photon energy. Compton scattering is thought to be the principal absorption mechanism for gamma rays in the intermediate energy range 100 keV to 10 MeV. Compton scattering is relatively independent of the atomic number of the absorbing material, which is why very dense metals like lead are only modestly better shields, on a per weight basis, than are less dense materials.
- Pair production: This becomes possible with gamma energies exceeding 1.02 MeV, and becomes important as an absorption mechanism at energies over about 5 MeV (see illustration at right, for lead). By interaction with the electric field of a nucleus, the energy of the incident photon is converted into the mass of an electron- positron pair. Any gamma energy in excess of the equivalent rest mass of the two particles (1.02 MeV) appears as the kinetic energy of the pair and the recoil nucleus. At the end of the positron's range, it combines with a free electron. The entire mass of these two particles is then converted into two gamma photons of at least 0.51 MeV energy each (or higher according to the kinetic energy of the annihilated particles).
The secondary electrons (and/or positrons) produced in any of these three processes frequently have enough energy to produce much ionization themselves.
Light interaction
[edit | edit source]High-energy (from 80 to 500 GeV) gamma rays arriving from far far-distant quasars are used to estimate the extragalactic background light in the universe: The highest-energy rays interact more readily with the background light photons and thus their density may be estimated by analyzing the incoming gamma-ray spectrums.[3]
Gamma ray production
[edit | edit source]Gamma rays are often produced alongside other forms of radiation such as alpha or beta. When a nucleus emits an α or β particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower energy state by emitting a gamma ray, in much the same way that an atomic electron can jump to a lower energy state by emitting infrared, visible, or ultraviolet light.

Gamma rays, x-rays, visible light, and radio waves are all forms of electromagnetic radiation. The only difference is the frequency and hence the energy of the photon s. Gamma rays are the most energetic. An example of gamma ray production follows.
Another example is the alpha decay of to form {; this alpha decay is accompanied by gamma emission. In some cases, the gamma emission spectrum for a nucleus (daughter nucleus) is quite simple, (e.g. while in other cases, such as with and, the gamma emission spectrum is complex, revealing that a series of nuclear energy levels can exist. The fact that an alpha spectrum can have a series of different peaks with different energies reinforces the idea that several nuclear energy levels are possible.

Because a beta decay is accompanied by the emission of a neutrino which also carries energy away, the beta spectrum does not have sharp lines, but instead is a broad peak. Hence from beta decay alone it is not possible to probe the different energy levels found in the nucleus.
In optical spectroscopy, it is well known that an entity which emits light can also absorb light at the same wavelength (photon energy). For instance, a sodium flame can emit yellow light as well as absorb the yellow light from a sodium vapor lamp. In the case of gamma rays, this can be seen in Mössbauer spectroscopy. Here, a correction for the energy lost by the recoil of the nucleus is made and the exact conditions for gamma ray absorption through resonance can be attained.
This is similar to the Franck Condon effects seen in optical spectroscopy.
Health effects
[edit | edit source]All ionizing radiation causes similar damage at a cellular level, but because rays of alpha particles and beta particles are relatively non-penetrating, external exposure to them causes only localized damage, e.g. radiation burns to the skin. Gamma rays and neutrons are more penetrating, causing diffuse damage throughout the body (e.g. radiation sickness, increased incidence of cancer) rather than burns. External radiation exposure should also be distinguished from internal exposure, due to ingested or inhaled radioactive substances, which, depending on the substance's chemical nature, can produce both diffuse and localized internal damage. The most biological damaging forms of gamma radiation occur in the gamma ray window, between 3 and 10 MeV, with higher energy gamma rays being less harmful because the body is relatively transparent to them. See cobalt-60.
Uses
[edit | edit source]
This property means that gamma radiation is often used to kill living organisms, in a process called irradiation. Applications of this include sterilizing medical equipment (as an alternative to autoclaves or chemical means), removing decay-causing bacteria from many foods or preventing fruit and vegetables from sprouting to maintain freshness and flavor.
Gamma-rays have the smallest wavelengths and the most energy of any wave in the electromagnetic spectrum. These waves are generated by radioactive atoms and in nuclear explosions. Gamma-rays can kill living cells, a fact which medicine uses to its advantage, using gamma-rays to kill cancerous cells.
Gamma-rays travel to us across vast distances of the universe, only to be absorbed by the Earth's atmosphere. Different wavelengths of light penetrate the Earth's atmosphere to different depths. Instruments aboard high-altitude balloons and satellites like the Compton Observatory provide our only view of the gamma-ray sky.
Due to their tissue penetrating property, gamma rays/X-rays have a wide variety of medical uses such as in CT Scans and radiation therapy. However, as a form of ionizing radiation they have the ability to effect molecular changes, giving them the potential to cause cancer when DNA is affected. The molecular changes can also be used to alter the properties of semi-precious stones, and is often used to change white topaz into blue topaz.
Despite their cancer-causing properties, gamma rays are also used to treat some types of cancer. In the procedure called gamma-knife surgery, multiple concentrated beams of gamma rays are directed on the growth in order to kill the cancerous cells. The beams are aimed from different angles to concentrate the radiation on the growth while minimizing damage to the surrounding tissues. (As an illustration of the radiation origin-process contributing to its name, a similar technique which uses photons from linacs rather than cobalt gamma decay, is called " Cyberknife ").
Gamma rays are also used for diagnostic purposes in nuclear medicine. Several gamma-emitting radioisotopes are used, one of which is technetium-99m. When administered to a patient, a gamma camera can be used to form an image of the radioisotope's distribution by detecting the gamma radiation emitted. Such a technique can be employed to diagnose a wide range of conditions (e.g. spread of cancer to the bones).
In the US, gamma ray detectors are beginning to be used as part of the Container Security Initiative (CSI). These US$ 5 million machines are advertised to scan 30 containers per hour. The objective of this technique is to screen merchant ship containers before they enter US ports.
Body response
[edit | edit source]After gamma-irradiation, and the breaking of DNA double-strands, a cell can repair the damaged genetic material to the limit of its capability. However, a study of Rothkamm and Lobrich has shown that the repairing process works well after high-dose exposure but is much slower in the case of a low-dose exposure.[4]
Risk assessment
[edit | edit source]The natural outdoor exposure in Great Britain ranges from Natural exposure to gamma rays is about 0.1 to 0.2 cSv per year, and the average total amount of radiation received in one year per inhabitant in the USA is 0.36 cSv. There is a small increase in the dose, due to naturally occurring gamma-radiation, around small particles of high atomic number materials in the human body caused by the photoelectric effect>
By comparison, the radiation dose from chest radiography is a fraction of the annual naturally occurring background radiation dose, and the dose from fluoroscopy of the stomach is, at most, 5 cSv on the skin of the back.
For acute full-body equivalent dose, 100 cSv causes slight blood changes; 200–350 cSv causes nausea, hair loss, hemorrhaging and will cause death in a sizable number of cases (10%–35%) without medical treatment; 500 cSv is considered approximately the LD50 (lethal dose for 50% of exposed population) for an acute exposure to radiation even with standard medical treatment; more than 500 cSv brings an increasing chance of death; eventually, above 750–1000 cSv, even extraordinary treatment, such as bone-marrow transplants, will not prevent the death of the individual exposed (see Radiation poisoning ).[clarification needed][citation needed]
For low dose exposure, for example among nuclear workers, who receive an average yearly radiation dose of 1.9 cSv,[clarification needed] the risk of dying from cancer (excluding leukemia) increases by 2 percent. For a dose of 10 cSv, that risk increase is at 10 percent. By comparison, risk of dying from cancer was increased by 32 percent for the survivors of the atomic bombing of Hiroshima and Nagasaki.[5]
The neutron is a subatomic particle with no net electric charge and a mass slightly larger than that of a proton. They are usually found in atomic nuclei. The nuclei of most atoms consist of protons and neutrons, which are therefore collectively referred to as nucleon s. The number of protons in a nucleus is the atomic number and defines the type of element the atom forms. The number of neutrons is the neutron number and determines the isotope of an element. For example, the abundant carbon-12 isotope has 6 protons and 6 neutrons, while the very rare radioactive carbon-14 isotope has 6 protons and 8 neutrons.
While bound neutrons in stable nuclei are stable, free neutrons are unstable; they undergo beta decay with a mean lifetime of just under 15 minutes (885.7±0.8 s).[6] Free neutrons are produced in nuclear fission and nuclear fusion. Dedicated neutron sources like research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments. Even though it is not a chemical element, the free neutron is sometimes included in tables of nuclides.[7] It is then considered to have an atomic number of zero and a mass number of one, and is sometimes referred to as neutronium.
Discovery
[edit | edit source]In 1931 Walther Bothe and Herbert Becker in Germany found that if the very energetic alpha particles emitted from polonium fell on certain light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. At first this radiation was thought to be gamma radiation, although it was more penetrating than any gamma rays known, and the details of experimental results were very difficult to interpret on this basis. The next important contribution was reported in 1932 by Irène Joliot-Curie and Frédéric Joliot in Paris. They showed that if this unknown radiation fell on paraffin, or any other hydrogen-containing compound, it ejected protons of very high energy. This was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such a hypothesis.
In 1932, James Chadwick performed a series of experiments at the University of Cambridge, showing that the gamma ray hypothesis was untenable. He suggested that the new radiation consisted of uncharged particles of approximately the mass of the proton, and he performed a series of experiments verifying his suggestion.[8]
The discovery of the neutron explained a puzzle involving the spin of the nitrogen-14 nucleus, which had been experimentally measured to be 1 h. It was known that atomic nuclei usually had about half as many positive charges as if they were composed completely of protons, and in existing models this was often explained by proposing that nuclei also contained some "nuclear electrons" to neutralize the excess charge. Thus, nitrogen-14 would be composed of 14 protons and 7 electrons to give it a charge of +7 but a mass of 14 atomic mass units. However, it was also known that both protons and electrons carried an intrinsic spin of 1⁄2 h, and there was no way to arrange 21 particles in one group, or in groups of 7 and 14, to give a spin of 1 h. All possible pairings gave a net spin of 1⁄2 h. However, when nitrogen-14 was proposed to consist of 3 pairs of protons and neutrons, with an additional unpaired neutron and proton each contributing a spin of 1⁄2 h in the same direction for a total spin of 1 h, the model became viable. Soon, nuclear neutrons were used to naturally explain spin differences in many different nuclides in the same way, and the neutron as a basic structural unit of atomic nuclei was accepted.
Intrinsic properties
[edit | edit source]Stability and beta decay
[edit | edit source]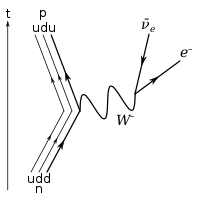
Under the Standard Model of particle physics, because the neutron consists of three quark s, the only possible decay mode without a change of baryon number is for one of the quarks to change flavour via the weak interaction. The neutron consists of two down quarks with charge -1⁄3 e and one up quark with charge +2⁄3 e, and the decay of one of the down quarks into a lighter up quark can be achieved by the emission of a W boson. By this means the neutron decays into a proton (which contains one down and two up quarks), an electron, and an electron antineutrino.
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 885.7±0.8 s (about 14 minutes, 46 seconds); therefore the half-life for this process (which differs from the mean lifetime by a factor of Natural logarithm) is 613.9±0.8 s (about 10 minutes, 14 seconds).[6] Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as beta decay:
Neutrons in unstable nuclei can also decay in this manner. However, inside a nucleus, protons can also transform into a neutron via inverse beta decay. This transformation occurs by emission of a antielectron (also called positron) and a neutrino:
The transformation of a proton to a neutron inside of a nucleus is also possible through electron capture:
- p+ + e → n + νe
Positron capture by neutrons in nuclei that contain an excess of neutrons is also possible, but is hindered because positrons are repelled by the nucleus, and quickly annihilate when they encounter electrons.
When bound inside of a nucleus, the instability of a single neutron to beta decay is balanced against the instability that would be acquired by the nucleus as a whole if an additional proton were to participate in repulsive interactions with the other protons that are already present in the nucleus. As such, although free neutrons are unstable, bound neutrons are not necessarily so. The same reasoning explains why protons, which are stable in empty space, may transform into neutrons when bound inside of a nucleus.
Electric dipole moment
[edit | edit source]The Standard Model of particle physics predicts a tiny separation of positive and negative charge within the neutron leading to a permanent electric dipole moment. The predicted value is, however, well below the current sensitivity of experiments. From several unsolved puzzles in particle physics, it is clear that the Standard Model is not the final and full description of all particles and their interactions. New theories going beyond the Standard Model generally lead to much larger predictions for the electric dipole moment of the neutron. Currently, there are at least four experiments trying to measure for the first time a finite neutron electric dipole moment.
Magnetic moment
[edit | edit source]Magnetic moment of a neutron is nonzero, unexpected from an electrically neutral particle. This indicates that neutron is a composite particle.
Anti-neutron
[edit | edit source]The antineutron is the antiparticle of the neutron. It was discovered by Bruce Cork in the year 1956, a year after the antiproton was discovered. CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles, so studying antineutrons yields provide stringent tests on CPT-symmetry. The fractional difference in the masses of the neutron and antineutron is 9±5×10−5. Since the difference is only about 2 standard deviations away from zero, this does not give any convincing evidence of CPT-violation.[6]
Neutron compounds
[edit | edit source]Dineutrons and tetraneutrons
[edit | edit source]The existence of stable clusters of 4 neutrons, or tetraneutrons, has been hypothesised by a team led by Francisco-Miguel Marqués at the CNRS Laboratory for Nuclear Physics based on observations of the disintegration of beryllium-14 nuclei. This is particularly interesting because current theory suggests that these clusters should not be stable.
The dineutron is another hypothetical particle.
Neutronium and neutron stars
[edit | edit source]At extremely high pressures and temperatures, nucleons and electrons are believed to collapse into bulk neutronic matter, called neutronium. This is presumed to happen in neutron star s.
Detection
[edit | edit source]The common means of detecting a charged particle by looking for a track of ionization (such as in a cloud chamber) does not work for neutrons directly. Neutrons that elastically scatter off atoms can create an ionization track that is detectable, but the experiments are not as simple to carry out; other means for detecting neutrons, consisting of allowing them to interact with atomic nuclei, are more commonly used. The commonly used methods to detect neutrons can therefore be categorized according to the nuclear processes relied upon, mainly neutron capture or elastic scattering. A good discussion on neutron detection is found in chapter 14 of the book Radiation Detection and Measurement by Glenn F. Knoll (John Wiley & Sons, 1979).
Neutron detection by neutron capture
[edit | edit source]A common method for detecting neutrons involves converting the energy released from such reactions into electrical signals. Certain nuclides have neutron deficit and therefore a high probability to absorb a neutron. Upon neutron capture, the compound nucleus emits more easily detectable radiation, for example an alpha particle, which is then detected. The nuclides 3He, 6Li, 10B, 233U, 235U, 237Np and 239Pu are useful for this purpose. These nuclides are rarely found in nature, but can be accumulated through processes such as isotopic enrichment.
The cross section for the process of neutron capture is much lower at high energies than at low energies. Therefore, the detection of neutrons by neutron capture requires a preceding slowing down of neutrons. For this purpose, a so-called moderator is used, typically a thick slab of polyethylene. Neutron detection according to the moderate-and-capture approach is not capable of measuring neutron energy, precise time of arrival, or direction of incidence, since this information is lost during moderation.
Neutron detection by elastic scattering
[edit | edit source]Neutrons can elastically scatter off nuclei, causing the struck nucleus to recoil. Kinematically, a neutron can transfer more energy to light nuclei such as hydrogen or helium than to heavier nuclei. Detectors relying on elastic scattering are called fast neutron detectors. Recoiling nuclei can ionize and excite further atoms through collisions. Charge and/or scintillation light produced in this way can be collected to produce a detected signal. A major challenge in fast neutron detection is discerning such signals from erroneous signals produced by gamma radiation in the same detector.
Fast neutron detectors have the advantage of not requiring a moderator, and therefore being capable of measuring the neutron's energy, time of arrival, and in certain cases direction of incidence.
Uses
[edit | edit source]The neutron plays an important role in many nuclear reactions. For example, neutron capture often results in neutron activation, inducing radioactivity. In particular, knowledge of neutrons and their behavior has been important in the development of nuclear reactors and nuclear weapons. The fissioning of elements like uranium-235 and plutonium-239 is caused by their absorption of neutrons.
Cold, thermal and hot neutron radiation is commonly employed in neutron scattering facilities, where the radiation is used in a similar way one uses X-rays for the analysis of condensed matter. Neutrons are complementary to the latter in terms of atomic contrasts by different scattering cross sections; sensitivity to magnetism; energy range for inelastic neutron spectroscopy; and deep penetration into matter.
The development of "neutron lenses" based on total internal reflection within hollow glass capillary tubes or by reflection from dimpled aluminum plates has driven ongoing research into neutron microscopy and neutron/gamma ray tomography.
A major use of neutrons is to excite delayed and prompt gamma rays from elements in materials. This forms the basis of neutron activation analysis (NAA) and prompt gamma neutron activation analysis (PGNAA). NAA is most often used to analyze small samples of materials in a nuclear reactor whilst PGNAA is most often used to analyze subterranean rocks around bore holes and industrial bulk materials on conveyor belts.
Another use of neutron emitters is the detection of light nuclei, particularly the hydrogen found in water molecules. When a fast neutron collides with a light nucleus, it loses a large fraction of its energy. By measuring the rate at which slow neutrons return to the probe after reflecting off of hydrogen nuclei, a neutron probe may determine the water content in soil.
Sources
[edit | edit source]Because free neutrons are unstable, they can be obtained only from nuclear disintegrations, nuclear reactions, and high-energy reactions (such as in cosmic radiation showers or accelerator collisions). Free neutron beams are obtained from neutron sources by neutron transport. For access to intense neutron sources, researchers must go to specialist facilities, such as the ISIS facility in the United Kingdom, which is currently the world's most intense pulsed neutron and muon source.[citation needed]
The neutron's lack of total electric charge makes it difficult to steer or accelerate them. Charged particles can be accelerated, decelerated, or deflected by electric or magnetic fields. These methods have little effect on neutrons beyond a small effect of an inhomogeneous magnetic field because of the neutron's magnetic moment. Neutrons can be controlled by methods that include Neutron moderation, reflection and velocity selection.
Protection
[edit | edit source]Exposure to free neutrons can be hazardous, since the interaction of neutrons with molecules in the body can cause disruption to molecules and atoms, and can also cause reactions which give rise to other forms of radiation (such as protons). The normal precautions of radiation protection apply: avoid exposure, stay as far from the source as possible, and keep exposure time to a minimum. Some particular thought must be given to how to protect from neutron exposure, however. For other types of radiation, e.g. alpha particles, beta particles, or gamma rays, material of a high atomic number and with high density make for good shielding; frequently lead is used. However, this approach will not work with neutrons, since the absorption of neutrons does not increase straightforwardly with atomic number, as it does with alpha, beta, and gamma radiation. Instead one needs to look at the particular interactions neutrons have with matter (see the section on detection above). For example, hydrogen rich materials are often used to shield against neutrons, since ordinary hydrogen both scatters and slows neutrons. This often means that simple concrete blocks or even paraffin-loaded plastic blocks afford better protection from neutrons than do far more dense materials. After slowing, neutrons may then be absorbed with an isotope which has high affinity for slow neutrons without causing secondary capture-radiation, such as lithium-6.
Hydrogen-rich ordinary water affects neutron absorption in nuclear fission reactors: usually neutrons are so strongly absorbed by normal water that fuel-enrichment with fissionable isotope is required. The deuterium in heavy water has a very much lower absorption affinity for neutrons than does protium (normal light hydrogen). Deuterium is therefore used in CANDU-type reactors, in order to slow (moderate) neutron velocity, to increase the probability of nuclear fission compared to neutron capture.
Production
[edit | edit source]Various nuclides become more stable by expelling neutrons as a decay mode; this is known as neutron emission, and happens commonly during spontaneous fission.
Cosmic radiation interacting with the Earth's atmosphere continuously generates neutrons that can be detected at the surface. Even stronger neutron radiation is produced at the surface of Mars where the atmosphere is thick enough to generate neutrons from cosmic ray spallation, but not thick enough to provide significant protection from the neutrons produced. These neutrons not only produce a Martian surface neutron radiation hazard from direct downward-going neutron radiation, but also a significant hazard from reflection of neutrons from the Martian surface, which will produce reflected neutron radiation penetrating upward into a Martian craft or habitat from the floor.[9]
Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing chain reaction. The intense neutron radiation can also be used to produce various radioisotopes through the process of neutron activation, which is a type of neutron capture.
Experimental nuclear fusion reactors produce free neutrons as a waste product. However, it is these neutrons that possess most of the energy, and converting that energy to a useful form has proved a difficult engineering challenge. Fusion reactors which generate neutrons are likely to create around twice the amount of radioactive waste of a fission reactor, but the waste is composed of neutron-activated lighter isotopes, which have relatively short (50–100 years) decay periods as compared to typical half lives of 10,000 years for fission waste, which is long primarily due to the long half life of alpha-emitting transuranic actinides.[10]
Neutron temperature
[edit | edit source]Thermal neutron
[edit | edit source]A thermal neutron is a free neutron that is Boltzmann distributed with kT = 0.0253 eV (4.0×10-21 J) at room temperature. This gives characteristic (not average, or median) speed of 2.2 km/s. The name 'thermal' comes from their energy being that of the room temperature gas or material they are permeating. (see kinetic theory for energies and speeds of molecules). After a number of collisions (often in the range of 10–20) with nuclei, neutrons arrive at this energy level, provided that they are not absorbed.
In many substances, thermal neutrons have a much larger effective cross-section than faster neutrons, and can therefore be absorbed more easily by any atomic nuclei that they collide with, creating a heavier — and often unstable — isotope of the chemical element as a result.
Most fission reactors use a neutron moderator to slow down, or thermalize the neutrons that are emitted by nuclear fission so that they are more easily captured, causing further fission. Others, called fast breeder reactors, use fission energy neutrons directly.
Cold neutrons
[edit | edit source]These neutrons are thermal neutrons that have been equilibrated in a very cold substance such as liquid deuterium. These are produced in neutron scattering research facilities.
Ultracold neutrons
[edit | edit source]Ultracold neutrons are produced by inelastically scattering cold neutrons in substances with a temperature of a few kelvins, such as solid deuterium or superfluid helium. An alternative production method is the mechanical deceleration of cold neutrons.
Fission energy neutron
[edit | edit source]A fast neutron is a free neutron with a kinetic energy level close to 2 M eV (20 T J/kg), hence a speed of 28,000 km/s. They are named fission energy or fast neutrons to distinguish them from lower-energy thermal neutrons, and high-energy neutrons produced in cosmic showers or accelerators. Fast neutrons are produced by nuclear processes such as nuclear fission.
Fast neutrons can be made into thermal neutrons via a process called moderation. This is done with a neutron moderator. In reactors, typically heavy water, light water, or graphite are used to moderate neutrons.
Fusion neutron
[edit | edit source]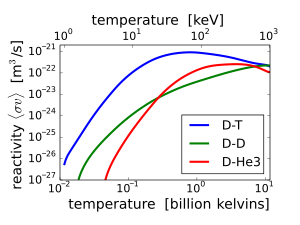
D-T (deuterium-tritium) fusion is the fusion reaction that produces the most energetic neutrons, with 14.1 MeV of kinetic energy and traveling at 17% of the speed of light. D-T fusion is also the easiest fusion reaction to ignite, reaching near-peak rates even when the deuterium and tritium nuclei have only a thousandth as much kinetic energy as the 14.1 MeV that will be produced.
14.1 MeV neutrons have about 10 times as much energy as fission neutrons, and are very effective at fissioning even non-fissile heavy nuclei, and these high-energy fissions produce more neutrons on average than fissions by lower-energy neutrons. 14.1 MeV neutrons can also produce neutrons by knocking them loose from nuclei. On the other hand, these very high energy neutrons are less likely to simply be captured without causing fission or spallation. For these reasons, nuclear weapon design extensively utilizes D-T fusion 14.1 MeV neutrons to cause more fission.
Other fusion reactions produce much less energetic neutrons. D-D fusion produces a 2.45 MeV neutron and helium-3 half of the time, and produces tritium and a proton but no neutron the other half of the time. D-3He fusion produces no neutron.
Intermediate-energy neutrons
[edit | edit source]
A fission energy neutron that has slowed down but not yet reached thermal energies is called an epithermal neutron.
Cross sections for both capture and fission reactions often have multiple resonance peaks at specific energies in the epithermal energy range. These are of less significance in a fast neutron reactor where most neutrons are absorbed before slowing down to this range, or in a well- moderated thermal reactor where epithermal neutrons mostly interact with moderator nuclei, not with either fissile or fertile actinide nuclides. However, in a partially moderated reactor with more interactions of epithermal neutrons with heavy metal nuclei, there are greater possibilities for transient changes in reactivity which might make reactor control more difficult.
Ratios of capture reactions to fission reactions are also worse (more captures without fission) in most nuclear fuels such as plutonium-239, making epithermal-spectrum reactors using these fuels less desirable, as captures not only waste the one neutron captured but also usually result in a nuclide which is not fissile with thermal or epithermal neutrons, though still fissionable with fast neutrons. The exception is uranium-233 of the thorium cycle which has good capture-fission ratios at all neutron energies.
High-energy neutrons
[edit | edit source]These neutrons have more energy than fission energy neutrons and are generated as secondary particles by particle accelerators or in the atmosphere from cosmic ray s. They can have energies as high as tens of joules per neutron.
References
[edit | edit source]- ↑ Hellemans, Alexander; Bunch, Bryan (1988). The Timetables of Science. Simon & Schuster. P.411. ISBN 0671621300.
- ↑ Shaw, R. W.; Young, J. P.; Cooper, S. P.; Webb, O. F. (1999). "Spontaneous Ultraviolet Emission from 233Uranium/229Thorium Samples". Physical Review Letters. 82 (6): 1109–1111. doi:10.1103/PhysRevLett.82.1109.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Bock, R. K. (2008-06-27). "Very-High-Energy Gamma Rays from a Distant Quasar: How Transparent Is the Universe?". Science (journal). 320 (5884): pp 1752–1754. doi:10.1126/science.1157087. ISSN 0036-8075. PMID 18583607.
{{cite journal}}:|pages=has extra text (help); Text "Science" ignored (help) - ↑ Rothkamm K. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proceedings of the National Academy of Science of the USA, 2003; 100 (9) : 5057-5062.
- ↑ IARC – Cancer risk following low doses of ionizing radiation – a 15-country study – http://www.iarc.fr/ENG/Units/RCAa1.html
- ↑ a b c Particle Data Group's Review of Particle Physics 2006
- ↑ http://www.nndc.bnl.gov/nudat2
- ↑ Chadwick, James (1932). "Possible Existence of a Neutron". Nature (journal). 129: 312. doi:10.1038/129312a0.
{{cite journal}}: Text "Nature" ignored (help) - ↑ http://www.physicamedica.com/VOLXVII_S1/20-CLOWDSLEY%20et%20alii.pdf
- ↑ [1] Nuclear power#Solid waste




