Radiation Oncology/Primary CNS Lymphoma
Jump to navigation
Jump to search
|
Front Page: Radiation Oncology | RTOG Trials | Randomized Trials | |
|
Non-Hodgkin lymphoma: Main Page | Randomized
| |
Epidemiology
[edit | edit source]- Median age of presentation for non-HIV related primary CNS lymphoma is 6th decade. HIV related presentation is related to stage of HIV.
- Incidence has increased 10-fold in past 30 years, and the increase cannot be totally explained by the advent of HIV.
- Has traditionally accounted for 1% of intracranial neoplasms in immunocompetent patients. The fraction now is estimated at 4%.
- Impacts two distinct populations:
- Immunocompromised (HIV, organ transplantation, immunodeficiency syndromes) - arise from EBV infection of B-cell lymphocytes
- Immunocompetent - no known etiology. B-cells have no normal role in the brain
- if HIV+ & surviving longer than 4 years, the frequency may be as high as 10% to 20% - though decreasing with HAART (Perez 2007)
- PCNSL frequently CSF+ (16% to 47% reported)
- Ocular involvement in 15% to 20% (vs Primary intraocular lymphoma spreads to the brain in 80%)
Pathology
[edit | edit source]- Usually B-cell histology
- T-cell variants account for <5% of PCNSL in developed countries; their behavior is very similar to B-cells
- 85% are either aggressive or highly aggressive variants of NHL
- PCNSL Collaborative Group; 2005 PMID 15800313 -- "Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group." (Shenkier TN, J Clin Oncol. 2005 Apr 1;23(10):2233-9.)
- Retrospective. 12 centers in 7 countries. 45 patients. Median age 60 years, 44% ECOG 0-1, 58% cerebral hemispheres, 26% deep lesions. LDH elevated in 32%, CSF elevated in 79%
- Outcome: DSS 2-year 51%, 5-year 17%
- Positive predictors: ECOG 0-1, MTX use
- Conclusion: Presentation and outcome similar to B-cell PCNSL
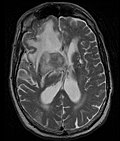
Anatomy
[edit | edit source]- Primary CNS Lymphoma
-
MRI T1 Axial
-
MRI T1 Coronal
-
MRI T1 Sagittal
-
MRI T2 Axial
- Restricted to brain, CSF, eyes, or rarely spinal cord
- Clinicopathologic variants include intracranial lesions, diffuse leptomeningeal or periventricular, vitreous, and spinal lesions.
- Intracranial primary CNS lymphoma are generally 75% supratentorial and 25% infratentorial.
- Primary CNS lymphoma is multifocal in 50% of AIDS-related variants, and multifocal in 25% of immunocompetent variants.
- MRI significantly underestimates extent of involvement; May appear focal on CT or MRI but parenchyma is typically diffusely involved.
- Intensely enhancing on MRI; may have a diffuse or “cotton wool” appearance on imaging.
- Classified as Stage IE NHL, because they are typically restricted to a single extranodal site
Prognostic Factors
[edit | edit source]- Multiple factors for poor prognosis: Age >60, ECOG PS >1, elevated LDH, elevated CSF protein, deep regions of the brain
- European Stratification: 5 variables above. Risk groups: 0-1 factors, 2-3 factors, 4-5 factors
- MSKCC Stratification: 2 variables, age and KPS
| Variables | Median OS | |
| RPA Class I | Age <=50 | 8.5 years |
| RPA Class II | Age >=50 & KPS >=70 | 3.2 years |
| RPA Class III | Age >=50 & KPS <70 | 1.1 years |
- MSKCC; 2006 (1983-2003) PMID 17116938 -- "Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model." (Abrey LE, J Clin Oncol. 2006 Dec 20;24(36):5711-5.)
- 338 patients analyzed. RPA analysis. External validation from 3 RTOG prospective trials
- RPA: Class I (patients <=50), Class II (patients >=50 and KPS >=70), Class III (>50 and KPS <70)
- MSKCC data: median OS 8.5 years vs. 3.2 years vs. 1.1 years
- Validation with RTOG data: median OS 5.2 years vs. 2.1 years vs. 0.8 years
- Conclusion: Simple, universally applicable
- International Extranodal Lymphoma Study Group; 2003 PMID 12525518 -- "Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience." (Ferreri AJ, J Clin Oncol. 2003 Jan 15;21(2):266-72.)
- 378 patients analyzed. Five variables developed for prognostic score: 1) age >60, ECOG >1, elevated LDH, high CSF protein, deep regions of the brain.
- 2-year OS: Score 0-1: 80%; Score 2-3: 48%; Score 4-5: 15%
- Conclusion: Prognostic score developed
- France; 1993 (1982-1991) PMID 8494713 -- "Multivariate analysis of prognostic factors in patients with non HIV-related primary cerebral lymphoma. A proposal for a prognostic scoring." (Blay JY, Br J Cancer. 1993 May;67(5):1136-41.)
- Retrospective. 41 patients with non-HIV PCNSL.
- Multivariate predictors: CSF protein >0.6 g/l, ECOG >2, Age >60
- Prognostic risk groups: median OS: good 54 months, intermediate 20 months, poor 4 months
- Conclusion: heterogenous disease, can be stratified
Treatment
[edit | edit source]Overview
[edit | edit source]- Debulking surgery typically does not play a role, unless patients have masses that threaten impending herniation
- Steroids should not be given, unless necessary to prevent herniation, since they can induce significant tumor regression and mask pathology. However, if steroids are given, immunostaining may still be possible even on dead cells
- Treatment for CNS lymphoma traditionally consisted of whole brain radiation therapy. The results were generally dismal. Although there is a high initial response rate to radiation, the vast majority of patients relapsed within the brain, and died of their disease. Median survival was 1 - 1.5 years, with 5-year OS ~5%
- Due to rarity of this disease, most studies are Phase II trials
- RTOG 8315 attempted to dose escalate brain treatment, but ultimately found unacceptably high rates of late side effects without significant improvement in overall survival. Ideal dose appears to be somewhere between 40 and 50 Gy; less than 40 Gy leads to worse survival, more than 50 Gy to worse neurotoxicity
- Even though CHOP is very effective in systemic NHL, its results in PCNSL have been disappointing
- Phase II single arm RTOG 9310 showed that chemo followed by RT consolidation had better outcomes than RT alone compared to historical controls. However, there was high neurotoxicity in patients >60 with combined modality therapy
- High dose methotrexate (HD-MTX) based chemo has become the backbone of primary treatment. Consolidative WBRT is too toxic for older patients (>60) and should be deferred. The role of consolidative WBRT in young patients (<60) is unclear, but appears to improve outcome. Median survival with sequential chemo-RT, as well as high dose chemo alone is now ~4 years
- Going forward, attempts are to optimize chemo regimens to defer neurotoxicity from consolidative WBRT
Current NCCN guidelines for treatment:
- For patient with KPS >40 and creatinine clearance >50, high dose MTX based chemotherapy is considered the standard treatment.
- If CSF cytology is positive, consider intrathecal chemotherapy.
- For patient with KPS <40 or creatinine clearance <50, WBRT to 45 Gy.
Current indications for radiation therapy:
- Consider as post-chemotherapy treatment in initial management of select younger patients (see RTOG 9310).
- Recurrent disease resistant to MTX based chemotherapy.
- Radiation should be avoided in patients >60 when possible.
Chemo+/-WBRT
[edit | edit source]- G-PCNSL-SG-1
- Multicenter, phase III noninferiority trial, 551 patients Non-AIDS randomized to received HD-MTX +/- WBRT. 318 pts treated per protocol included in analysis.
- WBRT : 45Gy/30F, 1.5Gy daily fraction
- Chemo: Initially MTX 4g/m2, but protocol amended in 2006 to give MTX 4g/m2 on day 1 and ifosfamide 1.5g/m2 on day 3-5
- Primary endpoint: overall survival, defined non-inferior as lower 95% CI not below 0.9. Designed to have 60% power.
- 2010 PMID 20970380 -- "High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial." (Thiel E, Lancet Oncol. 2010 Nov;11(11):1036-47.)
- Results: Median f/u: 4.2 years, included only 318/551 treated per protocol
- Per protocol analysis: median overall survival was 32.4mo(WBRT) vs 37.1mo, HR 1.06 (95% CI= 0.80-1.40, P=0.71). Median PFS was 18.3 mo(WBRT) vs 11·9 mo (P=0.15).
- Intent to treat analysis: no difference in OS, but WBRT improved PFS (HR=0.79, P=0.041). On subgroup analysis, WBRT improved PFS in patients without CR (HR=0.69, P=0.011), but not with CR (HR=0.79, P=0.21) after chemo.
- Toxicity: In 79 pt with sustained CR, more radiologic neurotoxicity 76% vs 46%, clinical neurotoxicity 49% vs 26%, in WBRT group
- 66 patients died after chemotherapy alone (all excluded from results analysis).
- Conclusion: No significant difference in OS. The PFS benefit of WBRT has to be weighed against the increased toxicity.
- Criticism: Ferrari Lancet Oncol 2011: PMID 21277546
- Did NOT meet preset primary endpoint that omitting WBRT had not inferior OS to giving WBRT.
- Used a per-protocol analysis, rather than intent to treat, which allows for selection biases.
- Extraordinarily high mortality with HD-MTX alone (12.5%), but all of these patients were excluded from analysis, making the results look better than they are
- Overall, many major protocol violations, and excluded 42% of the patients originally enrolled
- 66 pt died during chemo, 49 pt assigned do WBRT who did not get WBRT, 44 pt did not get salvage Ara-C in chemo-only arm with PR, 27 low to f/u, 22 did not have response criteria, 14 pt ineligible
- Only evaluated neurotoxicity outcomes in 79 patients, and clinical neurotoxicity endpoint not well defined. MMSE planned, but not done for most patients.
- Results: Median f/u: 4.2 years, included only 318/551 treated per protocol
Sequential Chemo-WBRT
[edit | edit source]- ANOCEF-GOELAMS PRECIS Study; 2019 (2008-2014) PMID 30785830 -- "Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study." (Houillier C, J Clin Oncol. 2019 Apr 1;37(10):823-833. doi: 10.1200/JCO.18.00306. Epub 2019 Feb 20.)
- Prospective, Phase II. 140 patients, 23 French centers. Untreated primary CNS lymphoma, age <=60. Induction R-MBVP / R-AraC, followed by randomization to Arm 1) Thiotepa / busulfan / cyclophosphamide vs Arm 2) WBRT 40/20.
- Outcome: 2-year PFS chemo 87% vs WBRT 63%, both exceeded threshold for efficacy, no p-value given
- Toxicity: Cognitive toxicity worse after WBRT (p = 0.004)
- Conclusion: Both consolidative chemo and WBRT are effective for patients <=60; efficacy favors chemotherapy. Specific risks should be considered.
- International Extranodal Lymphoma Study Group (IELSG), 2009 PMID 19767089 — "High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial." (Ferreri AJ, Lancet. 2009 Sep 18. [Epub ahead of print])
- Multicenter phase 2, randomized. 79 pts, age <=75. Randomized to 4 courses of MTX vs MTX + cytarabine. Following chemo, whole brain RT was given.
- CR in 18% (MTX) vs 46% (MTX+C); overall response rate 40% and 69%. Increased g3-4 heme toxicity, 15% vs 92%; treatment-related death in 1 pt vs 3 pts.
- Conclusion: The addition of high-dose cytarabine to high-dose methotrexate improved outcome with acceptable toxicity in pts 75 yrs and younger.
- RTOG 0227 Protocol -- "Phase I/II Study of Pre-Irradiation Chemotherapy with Methotrexate, Rituximab, and Temozolomide and Post-Irradiation Temozolomide for Primary Central Nervous System Lymphoma"
- Opened protocol: Rituximab + Temozolomide + Methotrexate -> WBRT 36/30 in 1.2 Gy BID -> Temozolomide
- EORTC 20962 (1997-2002)
- Phase II. 52 patients. Non-AIDS-related. MBVP (methotrexate (MTX) teniposide carmustine methylprednisolone) x 2 cycles with 2x IT-MTX, ara-C, and HC. Followed by 40 Gy WBRT.
- 2003 PMID 14597741 — "High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962." Poortmans PM et al. J Clin Oncol. 2003 Dec 15;21(24):4483-8. Median F/U 2.2 years.
- Outcome: median OS 3.8 years. 3-yr OS 58%.
- Toxicity: Grade 3-4 neutropenia 78%; 10% deaths treatment-related
- Conclusion: MBVP followed by WBRT has a high response rate, but is very toxic
- RTOG 9310 (1994-1997)
- Phase II prospective single arm trial. 98 patients. HIV-. Pre-radiation chemotherapy included MTX, vincristine, procarbazine and intraventricular MTX. RT was WBRT to 45 Gy; if ocular lymphoma then eyes 36/20. During study, protocol modified such that if CR after chemo, WBRT 36/30 BID. After RT, patients received hi-dose cytarabine.
- Primary outcome; 2002 PMID 12488408 — "Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10." DeAngelis LM et al. J Clin Oncol. 2002 Dec 15;20(24):4643-8.
- Outcome: Median OS 50.4 months in patients <60, and 21.8 months in patients >60 (overall 36.9 months); 5-year OS 32%, no difference if CR after chemo or not
- Toxicity: 15% patients experienced severe delayed neurologic toxicity.
- Conclusions: HD-MTX based regimen combined with consolidative RT is superior to RT alone when the overall survival data are compared to historical data. However, patients over age 60 had significant risk of late toxicity from the combined treatment.
- HFX-RT; 2005 PMID 16193393 -- "Secondary analysis of Radiation Therapy Oncology Group study (RTOG) 9310: an intergroup phase II combined modality treatment of primary central nervous system lymphoma." (Fisher B, J Neurooncol. 2005 Sep;74(2):201-5.)
- Retrospective. 82 patients, 66 standard WBRT (45/25) and 16 HFX (36/30)
- Neurotoxicity: Grade 5 (fatal): 10%, HFX 13% vs. RT 9% (NS); leukoencephalopathy later in HFX group. None of CR to chemo developed Grade 5 neurotoxicity
- Outcome: no difference
- Conclusion: HFX 25% lower BED dose, but no difference on PFS and OS. It delayed but not eliminate severe neurotoxicity
- MSKCC; 2006 (1992-1998) PMID 17008697 -- "Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma." (Gavrilovic IT, J Clin Oncol. 2006 Oct 1;24(28):4570-4.)
- Retrospective. 57 patients, average 65 years old, median KPS 70. Treated with HD-MTX x5 cycles + vincristine. Intra-Ommaya MTX, procarbazine. Then WBRT 45 Gy, followed by Ara-C 2 cycles. WBRT deferred for 68% patients >60. Median F/U 9.6 years for surviving patients
- Outcome: median OS 4.2 years, median PFS 10.7 years. Median OS for age >60 was 2.4 years, regardless of RT. Median OS for age <60 (most chemo-WBRT) not reached yet
- Conclusion: Excellent survival. For older patients, defer WBRT. For younger patients, deferring WBRT may significantly compromise disease control
- Trans-Tasman Radiation Oncology Group, TROG, (1991-97)
- Phase II. 46 pts. IV MTX (1 g/m2; days 1,8) + leucovorin. Pts with positive CSF received intrathecal Ara-C 60 mg twice/wk x 3 weeks, continued weekly x 3 after clearance of CSF. WBRT began on day 15, 45 Gy in 25 fx (180 cGy/d) with a boost to 50.4 Gy if localized involvement or whole brain dose to 50.4 Gy if diffuse.
- 2000 PMID 10653867, 2000 — "Phase II multicenter study of brief single-agent methotrexate followed by irradiation in primary CNS lymphoma." O'Brien P et al. J Clin Oncol. 2000 Feb;18(3):519-26.
- 2006 PMID 16198065, 2006 — "Combined-modality therapy for primary central nervous system lymphoma: Long-term data from a Phase II multicenter study." O'Brien PC et al. Int J Radiat Oncol Biol Phys. 2006 Feb 1;64(2):408-13.
- 5-yr OS 37%, MS 36m. 30% actuarial risk of neurotoxicity.
- Conclusion: combined modality treatment results in improved outcome compared to historical controls.
- CHOD-BVAM I/II (1990-1995)
- 2 simultaneous phase II prospective trials to evaluate reduced WBRT. 57 patients. Initial chemotherapy CHOD/BVAM. CR after chemo would place the patient into the BVAM II study (WBRT 30.6 Gy). A non-CR after chemo would place the patient into BVAM I (WBRT 45 Gy + 10 Gy boost to single lesion).
- 2002 PMID 11773174 -- "Importance of radiotherapy in the outcome of patients with primary CNS lymphoma: an analysis of the CHOD/BVAM regimen followed by two different radiotherapy treatments." (Bessell EM, J Clin Oncol. 2002 Jan 1;20(1):231-6.)
- Outcome: Reduced WBRT only predictor of relapse: standard WBRT 29% vs. reduced WBRT 70%. Age >60 only predictor for OS (RR 2.1). In young patients (<60), WBRT dose only predictor for OS: standard WBRT 92% vs. reduced WBRT 60% (SS)
- Toxicity: Incidence of RT-induced dementia rare in patients <60 years
- Conclusion: Reduction in WBRT dose in young patients after HD-MTX chemo results in increased risk of relapse and lower OS
Sequential WBRT-chemo
[edit | edit source]- NCCTG 96-73-51; 2006 (1998-2003) - PMID 16863926 — "Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: Results of North Central Cancer Treatment Group (NCCTG) 96-73-51." Laack NN et al. Int J Radiat Oncol Biol Phys. 2006 Aug 1;65(5):1429-39.
- 19 pts 70 years and older. After WBRT, 1 gram of methylprednisolone (Solu-Medrol) daily x 5 days, 30 days after RT. Then 1 g q28d until progression.
- 6 month OS of 33% at interim analysis; trial closed. Final results, longer OS 12.1 m and EFS 11.7 m in comparison to previous NCCTG trial of CHOP + high-dose ara-C + WBRT (OS 7.0m, EFS 4.0m).
- Conclusion: high dose methylprednisolone warrants further study
WBRT +/- CHOP
[edit | edit source]- MRC (1988-95)
- Phase III Closed early due to poor accrual, short of target population of 90 pts. 53 pts, treated with surgery, then randomized 2:1 to RT->CHOP vs RT alone. RT dose 40/20 WBRT + 14 Gy boost to 2cm margin. In RT-CHOP arm, RT was followed by CHOPx6.
- 2000 PMID 11002232 — "A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy." Mead GM et al. Cancer. 2000 Sep 15;89(6):1359-70. Median F/U 5 yrs.
- Outcome: No difference in OS adjusted for age/KPS
- Conclusion: inconclusive study due to poor accrual, but CHOP no clear role
WBRT alone
[edit | edit source]- RTOG 8315 (1983-87)
- Phase II prospective single arm trial; dose escalation. 41 patients. RT 40 Gy WBRT + 20 Gy boost to the tumor bed + 2cm margins
- 1992 PMID 1572835 — "Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315." Nelson DF et al. Int J Radiat Oncol Biol Phys. 1992;23(1):9-17.
- Outcome: median OS 12.2 months from diagnosis, 2-year OS 28%; Disease recurrence in the brain was 61%
- Prognostic factors: KPS >=70 median OS 23 months vs. 6 months (SS); age <60 23 months vs. 8 months (SS)
- Conclusion: Unreasonably high loco-regional recurrence, at the cost of significant leukoencephalopathy and cognitive impairment.
Chemotherapy alone
[edit | edit source]- German PCNSL-SG; 2005 PMID 15653703 -- "High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma." (Jahnke K, Ann Oncol. 2005 Mar;16(3):445-9. Epub 2005 Jan 14.)
- Phase IV prospective. 154 patients, 619 HD-MTX cycles, 4 g/m2 unless adjusted for decreased GFR, followed by LV rescue
- Toxicity: Grade >=3 was <10%; no difference if <60 or >=60
- Dose reduction: <60 18% vs. >60 44% (SS)
- Conclusion: HD-MTX safe treatment, regardless of age
- German deferred WBRT; 2003 (1995-2001) PMID 14597744 -- "Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy." (Pels H, J Clin Oncol. 2003 Dec 15;21(24):4489-95.)
- Phase II single arm. 65 patients. High dose MTX and Ara-C based chemotherapy (other chemo agents used include dexamethasone, vinca-alkaloids, ifosfamide, CTX). Intraventricular MTX, prednisolone, and Ara-C were also administered. Median F/U 2.2 years
- Outcome: Median TTF 1.7 years, median OS 4.2 years. If >60 years, median OS 2.8 years; not reached for <60 years
- Toxicity: 9% died of treatment related complications. 3% had permanent cognitive dysfunction.
- Conclusion: Primary chemo with HD-MTX and ARA-C is highly efficient, and outcomes comparable to chemo + RT
Intra-Arterial MTX-based chemo
- Multi-Institutional; 2009 (1982-2005) PMID 19451444 -- "Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy." (Angelov L, J Clin Oncol. 2009 Jul 20;27(21):3503-9. Epub 2009 May 18.)
- Phase II single arm. 4 institutions. 149 patients treated with Osmotic BBBD and IA infusion of methrotraxate + etoposide and cyclophosphamide or procarbazine. 46% age > 60, KPS < 70 in 42 %, CSF + in 7.4%
- Outcome: Overall response rate 81.9%, Median PFS was 1.8 years (95% CI, 1.3 to 2.8 years); 5-year PFS was 31%, 7-year PFS was 25%, Median OS was 3.1 years, 41% estimated 5-year survival, 25% estimated 8.5-year survival, Patients < 60 years median OS = 5.2 yrs and a 5-year survival = 52%, Patients > 60 years had median OS of 2.2 years and a 5-year survival = 30%
- Toxicity: Peri-procedural seizures in ~10%, Clinical strokes in 7.4% (Risk of permanent damage ~0.2%/BBBD-IA), Limited neurocognitive effects (per paper discussion, no data presented)
- Conclusion: BBBD and IA chemo results in excellent response and OS with minimal toxicity and allows for delay of WBRT. (Caution to use only in centers with experience in this technique)
Rituxan
[edit | edit source]- MSKCC; 2007 PMID 17947720 -- "Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma." (Shah GD, J Clin Oncol. 2007 Oct 20;25(30):4730-5.)
- Prospective. 30 patients (mean age 57, KPS 70). Treated with 5-7 cycles of induction chemo (R-MPV). If CR, dose-reduced WBRT 23.4 Gy; if PR, standard WBRT 45 Gy. Then 2 cycles Ara-C. Median F/U 3 years
- Outcome: 2-year OS 67%, PFS 57%. CR in 78% after 7 cycles, 44% after <=5 cycles
- Toxicity: Grade 3-4 neutropenia 43%, thrombocytopenia 36%, leukopenia 23%. Reduced WBRT in ~2/3 patients
- Conclusion: Immunochemotherapy is a promising approach; dose-reduced WBRT appears to eliminate risk of neurotoxicity without compromising control
- ASCO 2004: Abstract 1518 (2002-3) — "Combined immunochemotherapy with reduced dose whole brain radiotherapy (WBRT) for newly diagnosed patients with primary CNS lymphoma (PCNSL)." El Kamar FG.
- Phase II. 14 pts (of planned 30). Rituxan plus Methotrexate, Procarbazine and Vincristine (MPV). 23.4 Gy if CR after 5-7 cycles. 45 Gy if less than CR.
Salvage
[edit | edit source]- MGH; 2005 PMID 15735126 -- "Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma." (Nguyen PL, J Clin Oncol. 2005 Mar 1;23(7):1507-13.)
- Salvage whole brain RT: median 36 Gy (median 2 Gy/fx, most common 1.5 Gy/fx)
- 74% overall response rate (37% CR, 37% PR). Improved performance status in 44%. MS 10.9 mo.
- Conclusion: "For patients with PCNSL who experience treatment failure with methotrexate, WBRT provides high response rates (74%) and a median survival of 10.9 months. Age less than 60 years and response to WBRT predict post-WBRT survival. Modest rates of late neurotoxicity (15%) were seen and were associated with a total dose greater than 36 Gy."
Presentations
[edit | edit source]- ASCO 2004 - Video De Angelis LM - "Primary CNS Lymphoma: Update 2004"
Reviews
[edit | edit source]- MSKCC; 2007 - PMID 17591569 — "Primary central nervous system lymphoma." (Mohile NA, Semin Radiat Oncol. 2007 Jul;17(3):223-9.)
- MGH; 2006 - PMID 16525183 — "Primary CNS Lymphoma." (Batchelor T et al. J Clin Oncol. 2006 Vol 24, No 8 (March 10), 2006: pp. 1281-1288.)
- Yale; 2006 - PMID 16613654 — "Primary lymphoma of the nervous system." (Baehring JM et al. Cancer J. 2006 Jan;12(1):1.)