Problems In High School Chemistry/Inchem/Bondtypes
Chemical Bonds
Everyone has this one person in the world that makes them feel good. You are comfortable and more confident when you are around them. In other words, you bond with them. This also happens in chemistry. When an atom's valence electrons (outer shell) are not full, they are not stable. There are elements like hydrogen which only need 2 valence electrons (duet) and then there are elements like chlorine which needs 8 valence electrons (an octet). But when there is another atom, they can try and steal their valence electron, so they have a stable valence shell themselves (covalent bond) or they share it (ionic bond). Overall, we call them chemical bonds. In chemistry, there are three types of bonds: ionic bonds, metallic bonds, and covalent bonds. We are going to focus on ionic and covalent bonds today.
Electron Affinity (EA): how well an atom attracts electrons
Ionization Energy (IE): how well an atom holds onto his electrons
Nonmetals have high IE and AE which means they can attract electrons and hold onto them easily. Metals have low IE and EA which means they cannot attract nor hold onto them easily.
Ionic Bonds
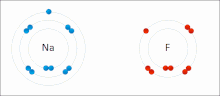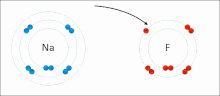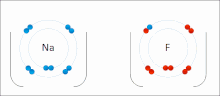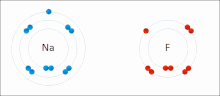
Made of nonmetals and metals. Nonmetals have high IE and high EA, which means they are strong enough to get an electron from the metal and keep their own electron, so they do not lose it. Meanwhile the metal is too “weak” to hold onto its electron let alone steal an electron from the nonmetal.
Covalent Bonds

Made of nonmetals. The non-metal atoms have a high IE and high EA. Both are strong enough to take an electron and to protect their own electron, but none is stronger than the other. Neither can steal electrons from the other, so they end up sharing electrons. The sharing of the electron pair between atoms keeps both atoms together, creating the covalent bond.